Table of Contents
1. How many protons, neutrons, and electrons are there in a neutral atom of Am-240 (americium-240)?
2. How to pronounce americium?
3. How many valence electrons does americium have?
4. What is the half-life of americium-242?
5. Where was americium discovered?
How many protons, neutrons, and electrons are there in a neutral atom of Am-240 (americium-240)?
In a neutral Americium-240 isotope there are 95 protons, 145 neutrons, and 95 electrons.
How to pronounce americium?
Try this AM-?-RISS-ee-?m (Americium).
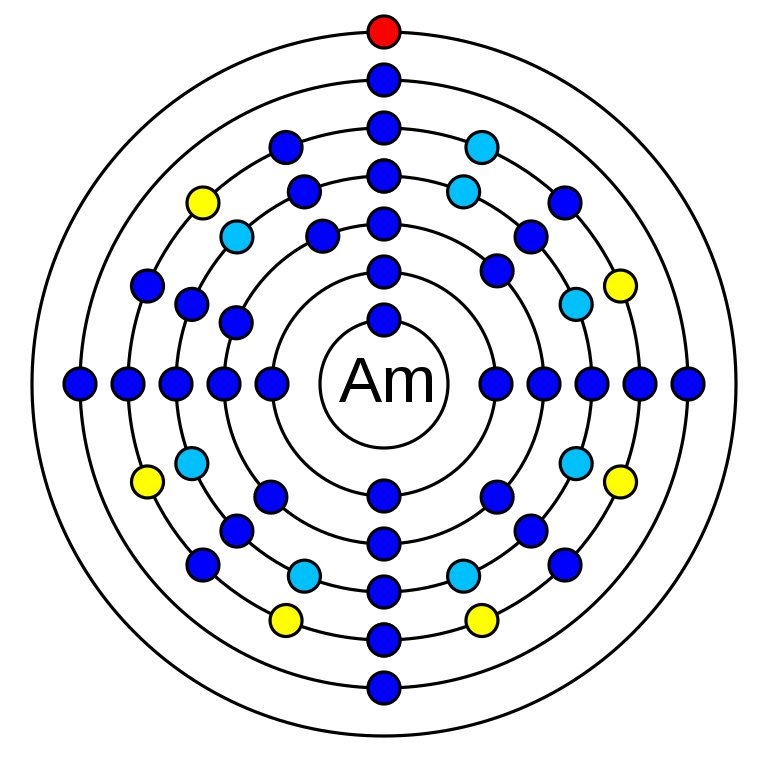
How many valence electrons does americium have?
There are two valence electrons in americium. These are in the 7s orbital.
What is the half-life of americium-242?
The half-life of Americium isotope Am-242 is 16.02 hours.
Where was americium discovered?
Americium was discovered by a group of scientists namely Glenn T. Seaborg, Leon O. Morgan, Ralph A. James, and Albert Ghiorso. It was produced in 1944 at the University of California, Berkeley using a cyclotron.
Where is americium found?
Americium is a highly radioactive element that can be found in a trace amount in Uranium minerals probably. Trace amounts can also be founds in places where nuclear tests were conducted. Small quantity is produced in the nuclear reactors mostly Americium-241 and Americium-243 isotopes. It can be produced in a 60-inch cyclotron which was first used in its preparation at the University of California, Berkeley.