Table of Contents
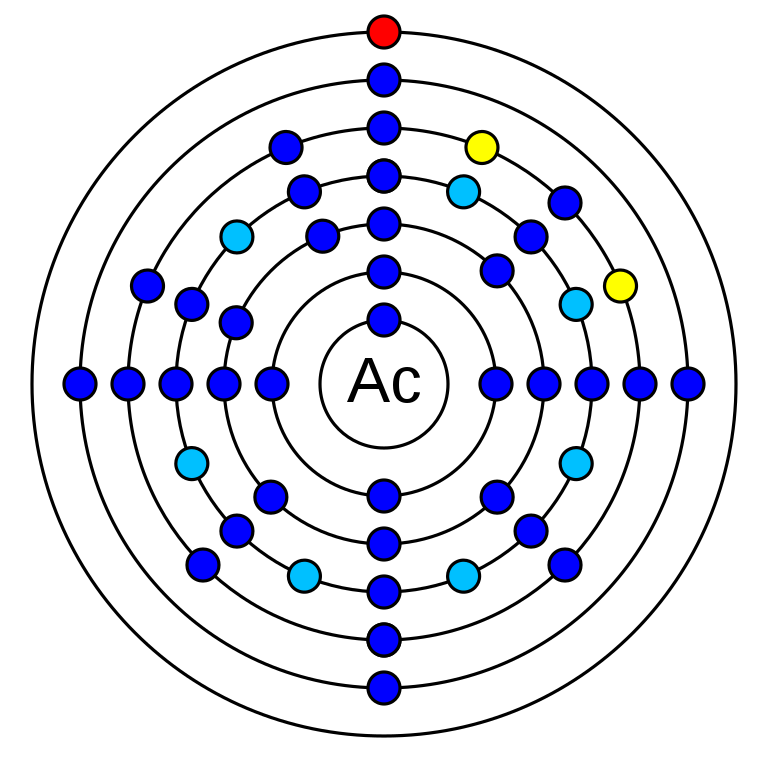
Actinium is a chemical element that has the atomic number 89. Its mass number is 227 and it is represented by the symbol Ac. It is the first member of the actinide series. It is an f-block element in period 7. It has 89 electrons and 138 neutrons. Its electronegativity is 1.1 (Pauling scale) and its oxidation state is +3. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 6d1 7s2. The energy required for 1st ionization is 499 kJ/mol and for 2nd is 1170 kJ/mol. Actinium has no stable isotope all are radioisotopes. There are 32 radioisotopes of Actinium.
At STP it is in solid-state and has a density of 10 g/cm3. Its melting point is 1323.15 K (1050 °C, 1922 °F) and its boiling point is 3500±300 K ?(3200±300 °C, ?5800±500 °F). Actinium is a soft radioactive metallic element with a silvery-white appearance. It quickly reacts with moisture and oxygen turning into the white colour of Actinium oxide.
It is found in uranium ores (Uraninite) in trace amounts. It can also be found in thorium ores. Actinium isotope Ac-227 is a product of Uranium decay of U-235. It can also be produced in nuclear reactors through the neutron irradiation of Ra-226.
Its scarcity makes its price very high and due to its radioactivity, it has no significant industrial use. But research is showing that it can be effective against leukaemia, lymphoma, breast, ovarian, neuroblastoma and prostate cancers.
Who discovered actinium?
The discovery of Actinium is a little controversial between André-Louis Debierne and Friedrich Oskar Giesel. André-Louis Debierne announced the discovery of a new element in 1899. Which he separated from the pitchblende residues. Debierne described it as similar to titanium. Debierne's results published in 1904 conflict with those reported in 1899 and 1900. But Debierne's chosen name for the new element was retained because it had seniority.
What is actinium used in?
Its scarcity makes its price very high and due to its radioactivity, it has no significant industrial use. But research is showing that it can be effective against leukaemia, lymphoma, breast, ovarian, neuroblastoma and prostate cancers.
Where is actinium found?
It is found in Uranium ores such as Uraninite and in thorium ores. It is produced as the radioactive decay of Uranium and thorium.