Table of Contents
3. How many valence electrons does astatine have?
5. How many neutrons does astatine have?
6. Astatine belongs to which element group?
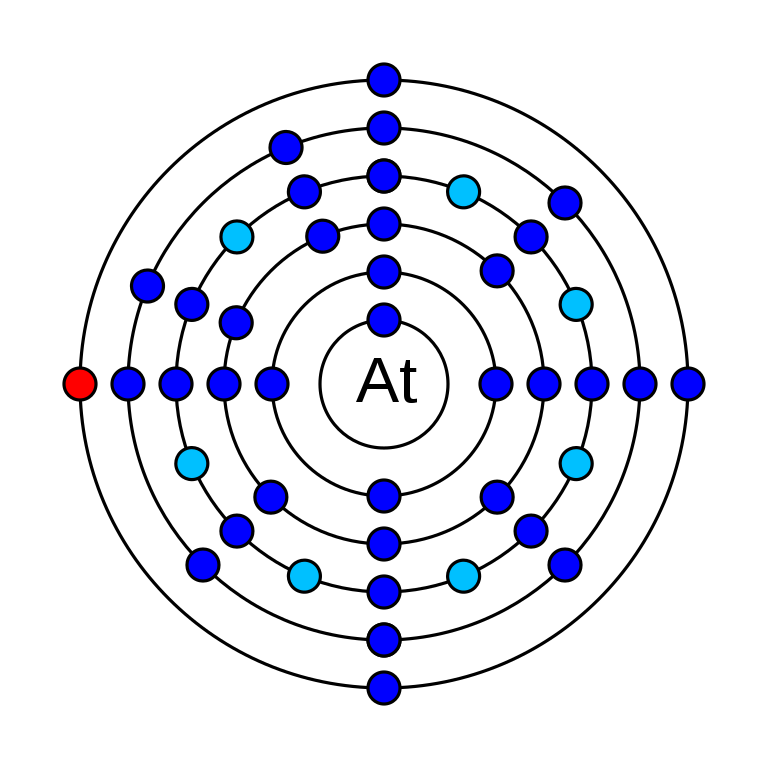
Astatine is a chemical element that has the atomic number 85. It has an average atomic mass of 209.98 g/mol and it is represented by the symbol At. It is a p-block element in group 17 and period 6 in the periodic table. In an electrically neutral atom of astatine, there are 85 electrons. The electronic configuration of Astatine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p5. Its oxidation states are −1, +1, +3, +5, +7 and its electro negativity is 2.2 (Pauling scale). Astatine has 39 isotopes and they are all radioisotopes. It has a very unstable nature and is also very rare in nature. 4 isotopes have been in nature. It was discovered by a group o scientists (Dale R. Corson, Kenneth Ross MacKenzie, Emilio Segrè) in the 1940s. It was produced in a cyclotron by bombarding bismuth-209 with alpha particles.
It is extremely radioactive almost all of its isotopes have half-lives of 8.1 hours or less. This makes it difficult to study, most of its physical properties are estimated and empirically derived from other halogens. Its solid structure is unknown.
Astatine is the rarest naturally occurring element. It is estimated that at any given time the total amount of astatine on earth is not exceeding from 1 gram. This makes it very difficult to study. The four naturally occurring isotopes of astatine are the result of the radioactive decay of thorium and uranium ores.
According to DR. Patricia Wallace Durbin;
''Astatine ... [is] miserable to make and hell to work with.''
Who discovered astatine?
Different scientists wrote on Astatine and claimed its discovery. But it was not proved or disproved by other scientists. This is because it is the rarest element in nature and has a very short half-life. A group of scientists Dale R. Corson, Kenneth Ross MacKenzie and Emilio Segrè produced it in cyclotron in 1940. It was synthesised by bombarding bismuth-209 with energetic alpha particles.
How many valence electrons does astatine have?
It has seven valence electrons. They are in 6s2 6p5 orbitals.
Where is astatine found?
Astatine has no stable isotope. All of its isotopes have a very short half-life which makes it difficult to mine ore extract. It is produced in a cyclotron by bombarding Bismuth-209 with alpha particles.
How many neutrons does astatine have?
It has 125 neutrons.
Astatine belongs to which element group?
It belongs to the halogen group. You can find it in group 17 and period 6 of the periodic table.