Table of Contents
2. Where was einsteinium discovered?
3. What does einsteinium look like?
4. How many neutrons does einsteinium have?
5. How many electrons does einsteinium have?
What is Einsteinium?
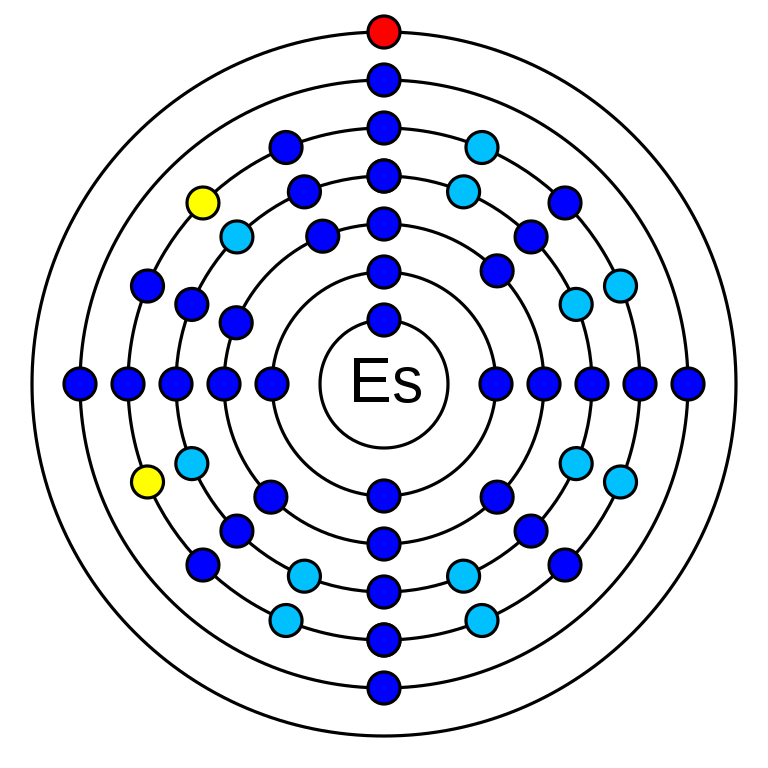
Einsteinium is a chemical element that has the atomic number 99. Its mass number is 252 and it is represented by the symbol Es. It is an actinide series member and it is in period 7 of the periodic table. Einsteinium is an f-block element and has 99 electrons, protons, and 153 neutrons. The electronic configuration of Einsteinium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f11 6d0 7s2. Its electro negativity is 1.3 (Pauling scale) and its oxidation states are +2, +3, +4. Einsteinium is a synthetic element all of its isotopes are radioisotopes. There are 18 known radioisotopes of Einsteinium.
At STP it is in solid-state and has a density of 8.84 g/cm3. It has a melting point of 1133 K ?(860 °C, ?1580 °F) and an estimated boiling point of 1269 K ?(996 °C, ?1825 °F). Einsteinium has a silvery metallic colour but glows blue in dark. Einsteinium was discovered by a team headed by Albert Ghiorso. It was discovered at Lawrence Berkeley National Laboratory in 1952. Einsteinium was named after Einstein.
It is possible that einsteinium was produced during Earth formation but it has been decayed. Because all isotopes of einsteinium have a very short half-life. All einsteinium is produced in labs and collected from high-power nuclear reactors, or in nuclear weapons tests. In 2008 it was detected in a star by the name Przybylski's Star or HD 101065 which is roughly 355 light-years away.
The Research Institute of Atomic Reactors (NIIAR) in Dimitrovgrad, Russia, and High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory in Tennessee, U.S are the two main laboratories that are producing a very trace amount of Einsteinium.
Other than scientific uses there is no use of any isotope of Einsteinium. Einsteinium-254 is used in the production of ultra heavy elements. In 1955 it was used in the production of mendelevium.
Where was einsteinium discovered?
Einsteinium was discovered in 1952 at Lawrence Berkeley National Laboratory. The element was discovered by a team headed by Albert Ghiorso.
What does einsteinium look like?
Einsteinium has a silvery metallic color and it glows blue in the dark.
How many neutrons does einsteinium have?
Einsteinium has 153 neutrons.
How many electrons does einsteinium have?
Einsteinium has 99 neutrons.