Table of Contents
2. How many protons does fermium have?
4. When was fermium discovered?
5. Where was fermium discovered?
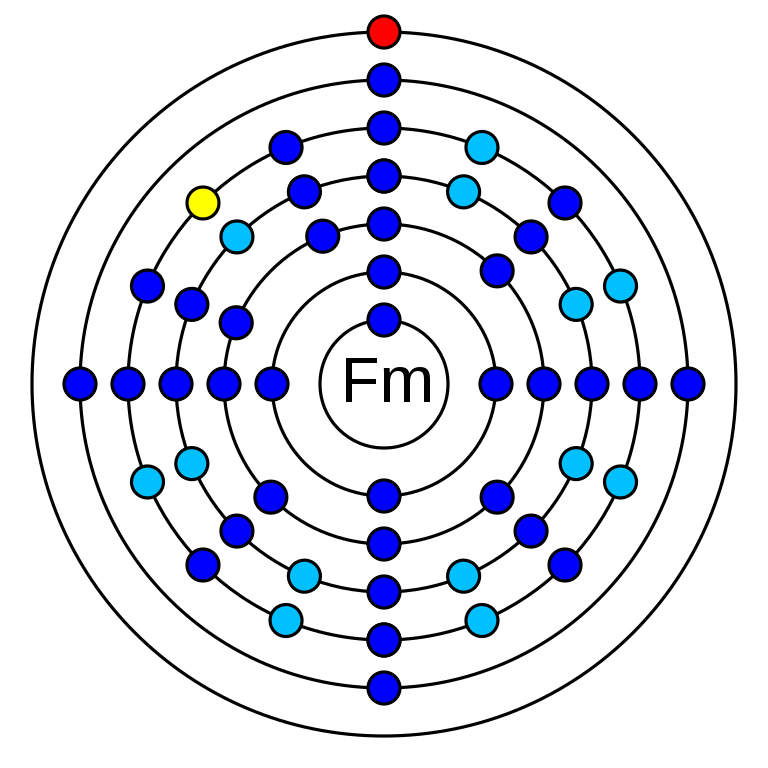
6. How many electrons are in fermium?
What is fermium?
Fermium is a chemical element that has an atomic number 100. Its mass number is 257 and it is represented by the symbol Fm. It is an f-block element in the actinide series and period 7 of the periodic table. Fermium has 100 electrons, protons and 157 neutrons. Its electronegativity is 1.3 (pauling scale) and its oxidation states are +2, +3. The electronic configuration of is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f12 6d0 7s2.
Fermium has 20 isotopes all are synthetic. Fm-257 isotope of Fermium has the longest half-life of 100.5 days.
Fermium physical properties are predicted. It is in solid-state and has a predicted density of 9.71 g/cm3. Its melting point is also predicted to be 1800 K ?(1527 °C, ?2781 °F).
A team of scientists headed by Albert Ghiorso discovered Fermium at the Lawrence Berkeley National Laboratory in 1952. It was named after nuclear physicist Enrico Fermi.
Fermium can be produced through the bombardment of neutrons on a lighter actinides. Almost all of Fermium are produced in 85 MW High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory in Tennessee, USA.
How many protons does fermium have?
Fermium has 100 protons in its nucleus.
Who discovered fermium?
Fermium was discovered by a group of scientists lead by Albert Ghiorso.
When was fermium discovered?
Fermium was discovered in 1952 at the Lawrence Berkeley National Laboratory.
Where was fermium discovered?
Fermium was discovered in Lawrence Berkeley National Laboratory (LBNL). Located in the hills of Berkeley, California.
How many electrons are in fermium?
In an electrically neutral atom of Fermium, there are 100 electrons.