Table of Contents
2. How many valence electrons does francium have?
3. When was francium discovered?
5. How many neutrons does francium have?
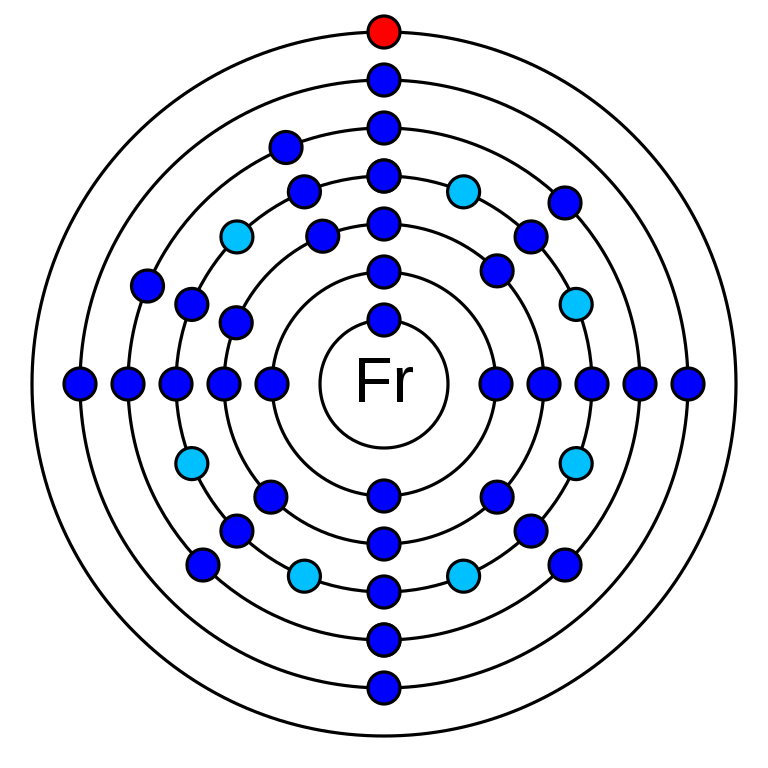
Francium is a Chemical element that has the atomic number 87. It has a relative atomic mass of 223.01 g/mol. It is an S-block element and classified as Alkali metal. In the periodic table, it is in group 1 and period 7. There are 87 electrons in an electrically neutral atom of Francium. The electronic configuration of Francium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s1.Its electronegativity is estimated to be 0.79 (Pauling scale) and its oxidation state is +1.
Francium is a highly radioactive element. It has no stable isotope but has 34 radioisotopes and metastable nuclear isomers. The longest-lived isotope of Francium is Fr-222 which has a half-life of about 22 minutes.
At STP Francium is in solid-state and has an estimated density of 2.48 g/cm3. Its boiling point is 950 K ?(677 °C, ?1251 °F) and its melting point is 300 K ?(27 °C, ?81 °F). It has similar properties to other elements of the alkali group.
It was first discovered and isolated by Marguerite Perey in 1939. She was a student of Marie Curie
Francium can be found in Uranium ores in trace amounts. Francium-223 is produced as the radioactive decay of Actinium-227.
Francium is very rare in nature which makes it not a suitable candidate for commercial uses. But used for research and teaching purposes. Studying and preparation of Francium required special equipment to handle it and specialized spectroscopy experiments.
How many valence electrons does francium have?
Francium is a member of the Alkali group and it has one valence electron. This is in the 7s orbital (7s1).
When was francium discovered?
Francium was discovered and isolated by French scientist Marguerite Perey in1939.
What is francium used for?
Francium is very rare and highly reactive. Naturally, there is no stable isotope of Francium. The longest-lived isotope of Francium is Francium-223 which has a half-life of 22 minutes. It is very difficult to handle and used for commercial purposes. Moreover, the rarity makes it very expensive to purify it needs special types of equipment. So there is no practical use of Francium except for teaching purposes etc.
How many neutrons does francium have?
Francium has 136 neutrons in its nucleus. Each isotope has a different number of Neutrons. Francium has some 34 isotopes and 7 metastable nuclear isomers.