Table of Contents
2. What colour is iridium metallic?
4. When was iridium discovered?
6. How many neutrons does iridium have?
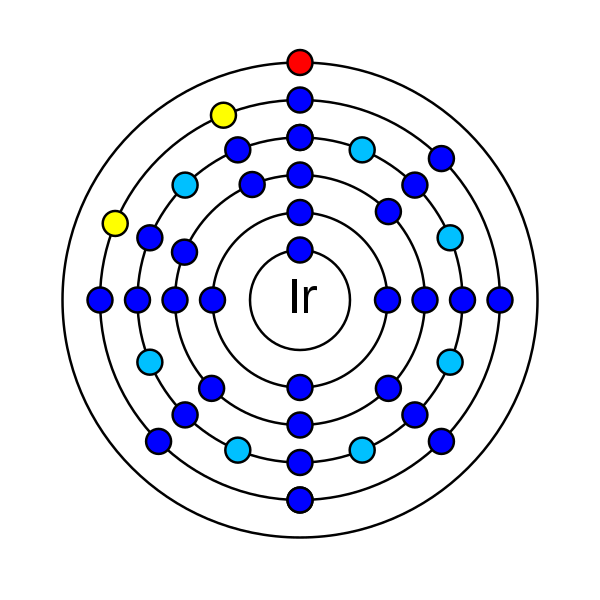
Iridium is a chemical element that has an atomic number (Z) 77. It has an atomic mass of 192.217 g/mol and it is represented by the symbol Ir. In the periodic table, it is in group 9 and period 6. It is a transition d-block element. There are 77 electrons, 77 protons and 115 neutrons in an electrically neutral atom of Iridium. The electronic configuration of iridium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d7 6s2. Its oxidation states are −3, −1, 0, +1, +2, +3, +4, +5, +6, +7, +8, +9 and its electronegativity is 2.20 (Pauling scale). The 1st ionization energy required is 880 kJ/mol and the 2nd is 1600 kJ/mol.
Iridium has two naturally occurring isotopes, Ir-191 and Ir-193. About 37 radioisotopes have been synthesized.
Iridium has a silvery-white colour. It has a very high corrosive resistance. It is not attacked by almost any acid, aqua regia, molten metals or silicates at high temperatures. But can be attacked by some salts at higher temperatures. At STP it is solid and has a density of 22.56 g/cm3. Its melting point is 2719 K ?(2446 °C, ?4435 °F) and its boiling point is 4403 K ?(4130 °C, ?7466 °F).
Iridium was discovered and first isolated by an English chemist Smithson Tennant in 1803.
Iridium is the ninth least abundant element in Earth's crust. It has an abundance of 0.001 ppm in crustal rock. Iridium is extremely rare it is collected as a byproduct from copper and nickel deposits and smelting. Its primary reserves are in South Africa ( Bushveld igneous complex) and also found in large deposits of copper-nickel deposits near Norilsk in Russia.
Iridium has high corrosion resistance and high melting point making it an important alloying agent. Osmium–Iridium is used in compass bearings and balances. Iridium based spark plugs are used in aviation. Iridium compounds are used as catalysts in the carbonylation of methanol producing acetic acid using the Cativa process. It is also used as a catalyst for the decomposition of hydrazine into nitrogen and ammonia. Iridium-192 a radioisotope of iridium is used in industrial γ-radiography for non-destructive testing of metals. International Prototype Metre and kilogram mass is 90% platinum and 10% iridium which is used as a standard for weight and length. Historically iridium was used in fountain pen nib tips.
What colour is iridium metallic?
Pure Iridium has a silvery-white metallic appearance. It is a hard brittle transition metal.
What is iridium used for?
Iridium has high corrosion resistance and a high melting point making it an important alloying agent.
Osmium–Iridium is used in compass bearings and balances.
Iridium based spark plugs are used in aviation.
Iridium compounds are used as catalysts in the carbonylation of methanol producing acetic acid using the Cativa process. It is also used as a catalyst for the decomposition of hydrazine into nitrogen and ammonia.
Iridium-192 a radioisotope of iridium is used in industrial γ-radiography for non-destructive testing of metals.
International Prototype Metre and kilogram mass is 90% platinum and 10% iridium which is used as a standard for weight and length.
Historically iridium was used in fountain pen nib tips.
When was iridium discovered?
It was discovered in 1803 by an English chemist Smithson Tennant.
How rare is iridium?
Iridium is the ninth least abundant stable element. It has an average abundance of 0.001 ppm in crustal rock. Gold is 40 times more abundant and silver is 80 times than Iridium. In meteorites its concentration increase up to 0.5ppm. The Willamette meteorite has an average of 4.7 ppm Iridium.
How many neutrons does iridium have?
A single atom of Iridium has 115 neutrons in its nucleus.