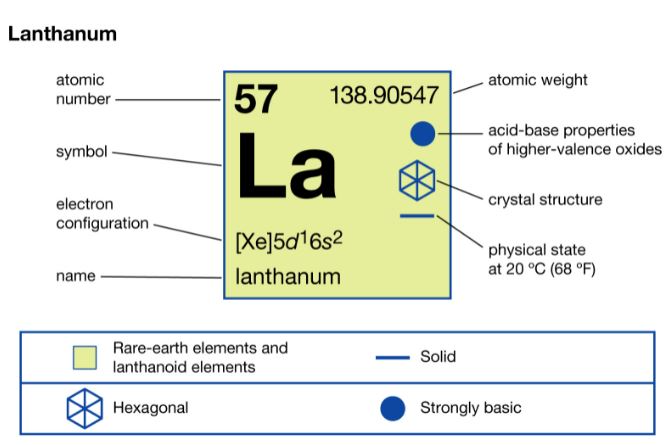
Lanthanum is an element that has an atomic number 57. Its atomic mass is 138.905 g/mol and it is represented by the symbol La. Lanthanum in pure form has a silvery-white colour. It is a soft metal when highly pure. It changes colour when exposed to air. In the periodic table, it is the first element of the lanthanide series and period 6.
There are 82 neutrons, 57 electrons and protons in an atom of Lanthanum. The electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2. There are three electrons in its valency. The oxidation states of Lanthanum is 0, +1, +2, +3. Its electronegativity is 1.10 (Pauling scale).
Lanthanum is solid at STP and has a density of 6.162 g/cm3. It is melting at a temperature of 1193 K ?(920 °C, ?1688 °F) and boils at 3737 K ?(3464 °C, ?6267 °F).
Lanthanum has one stable naturally occurring isotope La-139 with an abundance of 99.911%. It was discovered by Swedish chemist Carl Gustaf Mosander in 1838.
Lanthanum is found in minerals like bastnäsite and monazite. It has an average abundance of 39 mg/kg in the Earth's crust.
When was lanthanum discovered?
Lanthanum was discovered in 1838 by a Swedish chemist Carl Gustaf Mosander.
Where is lanthanum found?
Of all lanthanides, Lanthanum is the third most abundant. It is estimated that the Earth's crust has up to 39 mg/kg of Lanthanide. It is found in minerals like bastnäsite and monazite. Lanthanum is not rare as it is thought and called rare earth metal.
How many valence electrons does lanthanum have?
Lanthanum has three valence electrons. One is in 5d1 orbit and two are in 6s2 orbital. The electronic configuration of Lanthanum is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2.