Table of Contents
2. What is neptunium used for?
4. What is the electron configuration of neptunium?
What is neptunium?
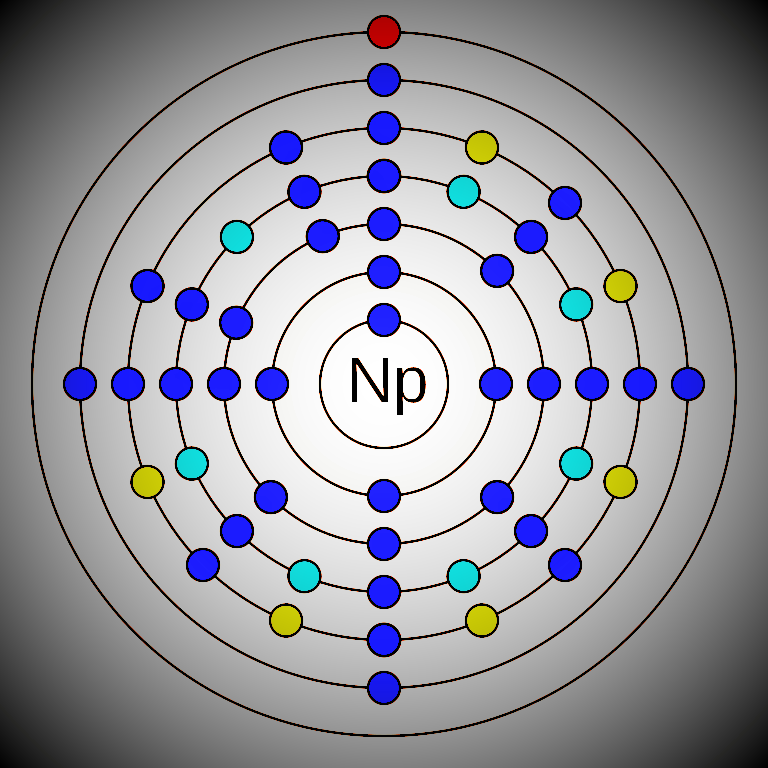
Neptunium is a chemical element that has the atomic number 93. Its mass number is 237 and it is represented by the symbol Np. Neptunium is a radioactive f-block element in the actinide series and in period 7 of the periodic table. It has 93 electrons and 144 neutrons.The electronic configuration of Neptunium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f4 6d1 7s2. This configuration is not expected by the Aufbau principle. This is because the electrons in 5f, 6d, and 7s subshells have similar electron energies. The oxidation states of Neptunium are +2, +3, +4, +5, +6, +7 and its electronegativity is 1.36 (Pauling scale). Two isotopes of neptunium are found in nature in a very trace amount (Np-237 and Np-239). Overall it is considered an artificial element with some Twenty-five radioisotopes. Neptunium was discovered by Edwin McMillan and Philip H. Abelson in the 1940s.
Neptunium has a silvery color and is a hard metal. At STP it has a density of 20.45 g/cm3. Its melting point is 917.15 K ?(644 °C, ?1192.2 °F) and its boiling point is 4175.15 K (3902 °C, 7055.6 °F).
Neptunium is hard to find in nature. There are just two isotopes that can be found in nature in a very trace amount. It can be produced in the cyclotron by bombarding Uranium. It can be separated in trace amounts from used nuclear fuel.
Its rarity is preventing it from wide use and application. Theoretically, it can be used in weapons of mass destruction and in nuclear reactors for energy purposes. It can be used for the detection of high-energy neutrons and in smoke detectors.
What is neptunium used for?
Neptunium is very rare in nature. This makes it expensive for ordinary uses. Theoretically, Neptunium is capable to be used in nuclear weapons and in nuclear reactors for energy consumption. Neptunium is fissionable and capable of sustaining a nuclear fission chain reaction. Neptunium-237 is used for detection purposes such as in devices for detecting high-energy (MeV) neutrons.
Who discovered neptunium?
Neptunium was discovered by Edwin McMillan and Philip H. Abelson in 1940.
What is the electron configuration of neptunium?
The electronic configuration of Neptunium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f4 6d1 7s2. This configuration is not expected by the Aufbau principle. This is because the electrons in 5f, 6d, and 7s subshells have similar electron energies.