Table of Contents
4. How many valence electrons does osmium have?
5. When was osmium discovered?
7. How many protons does osmium have?
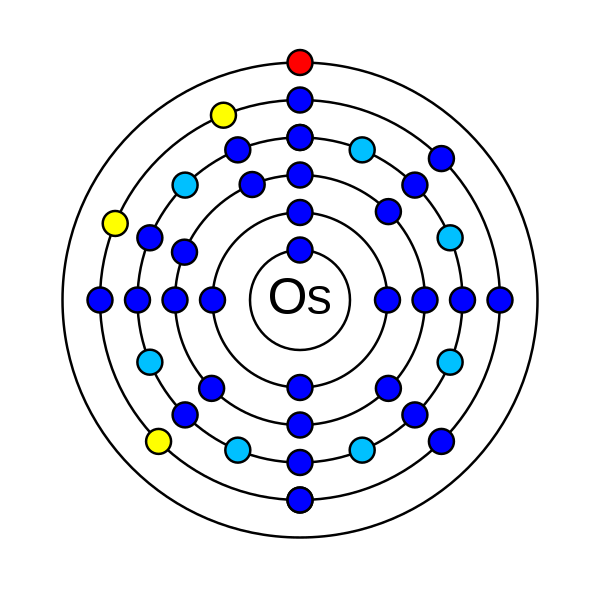
Osmium is a chemical element that has an atomic number (Z) 76. Its atomic mass is 190.23 g/mol and it is represented by the symbol Os. In the periodic table, it can be found in group 8 and period 6. It is a d-block transition metal. In an electrically neutral atom of Osmium, there is 76 electrons, 76 protons and 114 neutrons. The electronic configuration of Osmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d6 6s2. The electronegativity of Osmium is 2.2 (Pauling scale). The oxidation states of osmium ranging from −2, −1, 0, +1, +2, +3, +4, +5, +6, +7, to +8.
Osmium has seven naturally occurring isotopes. In five out of seven are stable the remaining has a long half-life. Osmium-192 is stable and the most abundant of all of them. Osmium has some 30 artificial radioisotopes.
The osmium metal has a brittle, bluish-white appearance. At STP it is solid and has a density of 22.59 g/cm3. It is 1 1⁄6 times as dense as gold. It has the fourth-highest melting point after carbon, tungsten and Rhenium. The melting point of Osmium is 3306 K ?(3033 °C, ?5491 °F) and its boiling point is 5285 K ?(5012 °C, ?9054 °F). It has very low compressibility and very difficult to change solid osmium in different shapes. Osmium was discovered and first isolated by English chemist Smithson Tennant in 1803.
Osmium is the least abundant metal it has an average abundance of 50 parts per trillion in the earth's crust. Mostly it is found in platinum ores. It is found in osmiridium an alloy of Osmium and iridium as well as in copper and nickel deposits in trace amounts. The largest reserves of Osmium are in South Africa (Bushveld Igneous Complex), Norilsk in Russia and Sudbury Basin in Canada. These are the main sources of Osmium.
Its alloys are used in different materials. osmiridium is a very hard alloy of osmium that is used in the tips of fountain pens. Osmium tetraoxide is has been used in fingerprint detection as well as for optical and electron microscopy.
How heavy is osmium?
At room temperature, it has a density of 22.59 g/cm3. For comparison, it is 23 times as dense as water, and 11⁄6 times as dense as gold. It has very low compressibility. Its bulk modulus is extremely high between 395-462 Gpa, a rival of the diamond which is 443 GPa.
Where is osmium found?
Osmium has an average concentration of about 50 ppt in the earth's crust. It is a rare metal found in trace amounts in platinum ores. It is found in nickel and copper deposits which are removed as a byproduct. The largest reserves are in South Africa ( Bushveld Igneous Complex) other can be found in copper-nickel deposits near Norilsk in Russia and in Sudbury Basin in Canada which is also a significant source of osmium.
How many valence electrons does osmium have?
In an electrically neutral atom of osmium, there are 76 electrons. The electronic configuration of Osmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d6 6s2. There are 8 electrons in valence shell of osmium.
When was osmium discovered?
Osmium was discovered in 1803 by English chemist Smithson Tennant.
How rare is osmium?
It has an average concentration of 50 ppt in the earth's crust. In the universe, it is 0.6 ppb and it is the rarest precious metal.
How many protons does osmium have?
In an electrically neutral atom of osmium, there are 76 protons and 114 neutrons in its nucleus. The atomic mass of Osmium is 190.23 g/mol.