Table of Contents
2. What is platinum used for? 3
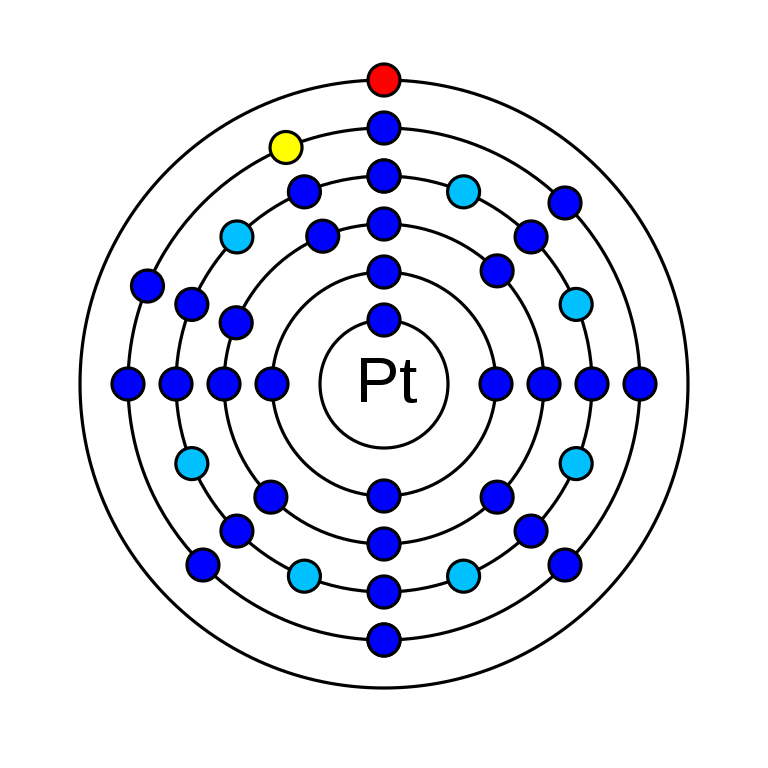
Platinium is a chemical element that has the atomic number 78. It has an atomic mass of 195.084 g/mol and it is represented by the symbol Pt. In the periodic table, it is in group 10 period 6. It is a d-block element. In an electrically neutral atom of platinum, there are 78 electrons and protons as well as 117 neutrons in its nucleus. The electronic configuration of platinum is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d9 6s1. Its oxidation states are −3, −2, −1, 0, +1, +2, +3, +4, +5, +6 and its electronegativity is 2.28 (Pauling scale). The ionization energy required for the 1st electron is 870 kJ/mol and for the 2nd electron is 1791 kJ/mol. There are five stable naturally occurring isotopes of Platinium and one radioisotope. There are 34 synthetic radioisotopes of platinum.
Platinium is a ductile, and malleable metal even more ductile than gold, silver or copper. It has excellent resistance to corrosion. It has a silvery-white colour. Platinum is a noble metal that can not be dissolved by acids like sulfuric acid and Hydrochloric acid. It can be dissolved by hot aqua regia. At STP it is solid and has a density of 21.45 g/cm3. It has a melting point of about 2041.4 K ?(1768.3 °C, ?3214.9 °F) and a boiling point of 4098 K ?(3825 °C, ?6917 °F).
It was discovered by Spanish chemist Antonio de Ulloa in1735.
Platinium is an extremely rare element with an average concentration of about 0.005 ppm in Earth's crust. It can be found in alluvial deposits and some nickel-copper ores which are collected as a byproduct. Another key source is sperrylite and cooperite. The largest platinum deposits are in India, South Africa, Russia and Canada.
Platinium is used as a catalyst in chemical reactions. In automobiles, it is used as a catalytic converter. In the petroleum industry, it is used in the conversion of Naphtha into higher octane gasoline. It is used in the hydrogenation of vegetable oils. The international prototype of the meter and kilogram is made of platinum and iridium alloy. It was used as a standard from 1799. Until May 2019. The bar and kilogram are made of 90% platinum and 10% Iridium. Standard Platinum Resistance Thermometer (SPRT) is used to define the International Temperature Scale of 1990 (ITS-90). Platinium is used in standard hydrogen electrodes because of its corrosive resistance nature and electrode potential. It is used as an alloying agent for medical instruments, dental prostheses and electrical contacts.
What is platinum used for?
- Platinium is used as a catalyst in chemical reactions.
- In automobiles, it is used as a catalytic converter.
- In the petroleum industry, it is used in the conversion of Naphtha into higher octane gasoline.
- It is used in the hydrogenation of vegetable oils.
- The international prototype of the meter and kilogram is made of platinum and iridium alloy. It was used as a standard from 1799. Until May 2019. The bar and kilogram are made of 90% platinum and 10% Iridium.
- Standard Platinum Resistance Thermometer (SPRT) is used to define the International Temperature Scale of 1990 (ITS-90).
- Platinium is used in standard hydrogen electrodes because of its corrosive resistance nature and electrode potential.
- It is used as an alloying agent for medical instruments, dental prostheses and electrical contacts.
What colour is platinum?
Pure platinum has silvery-white Colour. It is a dense, malleable, ductile, highly unreactive and precious metal.
Where is platinum found?
Platinium can be found in alluvial deposits, sperrylite, serpentinite and cooperite ores. Places, where deposits of Platinium, are in the Ural Mountains, Russia, Sudbury Basin deposit in Canada and Sudbury Basin deposit in South Africa.