Table of Contents
3. What does plutonium look like?
4. What is plutonium used for?
6. What is the atomic number for plutonium?
7. How many neutrons does plutonium have?
What is plutonium?
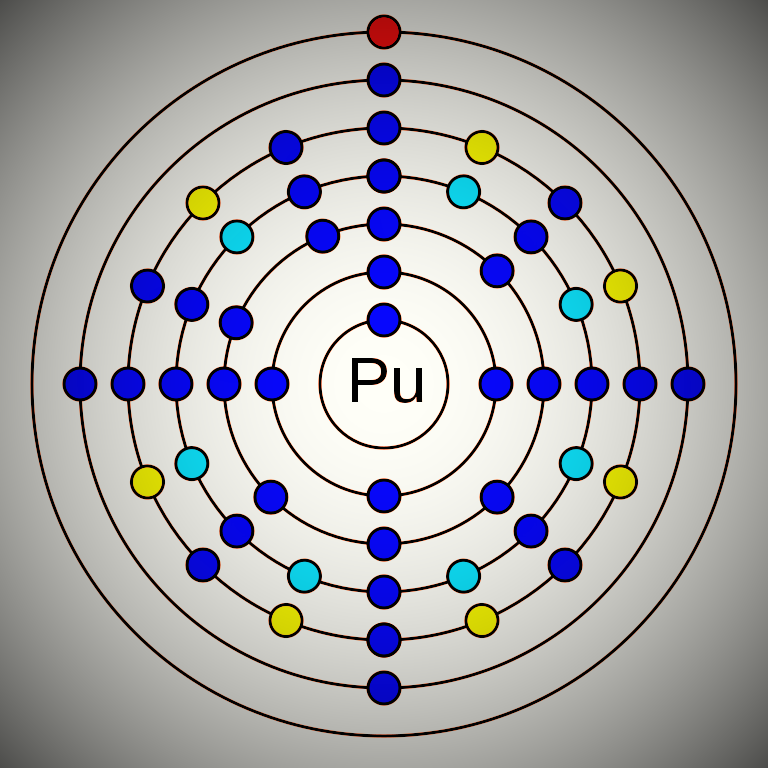
Plutonium is a chemical element that has the atomic number 94. Its mass number is 244 and it is represented by the symbol Pu. It is an f-block element. In the periodic table, it is in the actinide series, and in period 7. Plutonium has 94 electrons and 150 neutrons. The electronic configuration of Plutonium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f6 6d0 7s2. Its electronegativity is 1.28 (Pauling scale) and its oxidation states are +2, +3, +4, +5, +6, +7, +8. Plutonium is a radioactive element and very rare in nature. Some 4 isotopes can be found in a very trace amount in nature. The longest-lived isotope is plutonium-244.
Plutonium has a silvery-white color which tarnishing to dark grey on exposure to air. At STP it has a density of 19.85 g/cm3. Its melting point is 912.5 K ?(639.4 °C, ?1182.9 °F) and its boiling point is 3505 K ?(3228 °C, ?5842 °F). It has six allotropes at high pressure it makes seven.
Plutonium was discovered by a group of scientists namely Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan in 1940-1941. It was named after the god of the underworld Pluto.
Four isotopes of plutonium can be found in nature in a very trace amount. They are plutonium-238, plutonium-239, plutonium-240, and plutonium-244. It is produced from uranium by bombarding it with neutrons. It can also be produced by bombarding uranium-235 with deuterons. It can be accumulated from nuclear waste in a very small amount.
Plutonium's main uses were in nuclear weapons. Due to its rarity, it is very difficult to extract making it expensive for other uses and dangerous because of its radioactivity. Another main use is in nuclear reactors for energy production. It is producing a large amount of thermal energy with low gamma radiation. That is why it is used in radioisotope thermoelectric generators and radioisotope heater units in space probes and rovers such as Cassini, Voyager, Galileo Curiosity, and Perseverance.
Who discovered plutonium?
In 1934 a group of scientists led by Enrico Fermi from the university of Rome reported that they had discovered element No 94. But the sample they produced contained products of nuclear fission which were barium and krypton. Plutonium-238 was isolated in 1940-41 by a group of scientists at Berkeley Radiation Laboratory lead by Glenn T. Seaborg.
What does plutonium look like?
Plutonium has a metallic silvery white which turns into dark gray when exposing to air.
What is plutonium used for?
Plutonium's main uses were in nuclear weapons. Due to its rarity, it is very difficult to extract making it expensive for other uses and dangerous because of its radioactivity. Another main use is in nuclear reactors for energy production. It is producing a large amount of thermal energy with low gamma radiation. That is why it is used in radioisotope thermoelectric generators and radioisotope heater units in space probes and rovers such as Cassini, Voyager, Galileo Curiosity, and Perseverance, etc.
How heavy is plutonium?
The density of plutonium is 19.85 g/cm3. For comparison, water has a density of 1 g/cm3 and gold has 19.30 g/cm3.
What is the atomic number for plutonium?
The atomic number of Plutonium is 94 and it is represented by the symbol Pu.
How many neutrons does plutonium have?
Plutonium has 150 neutrons.