Table of Contents
2. What type of radiation is emitted when polonium-212 forms lead-208?
3. When was polonium discovered?
4. What does the 218 in polonium-218 represent?
5. To what element does polonium-208 (atomic number 84) decay when it emits an alpha particle?
6. How many valence electrons does polonium have?
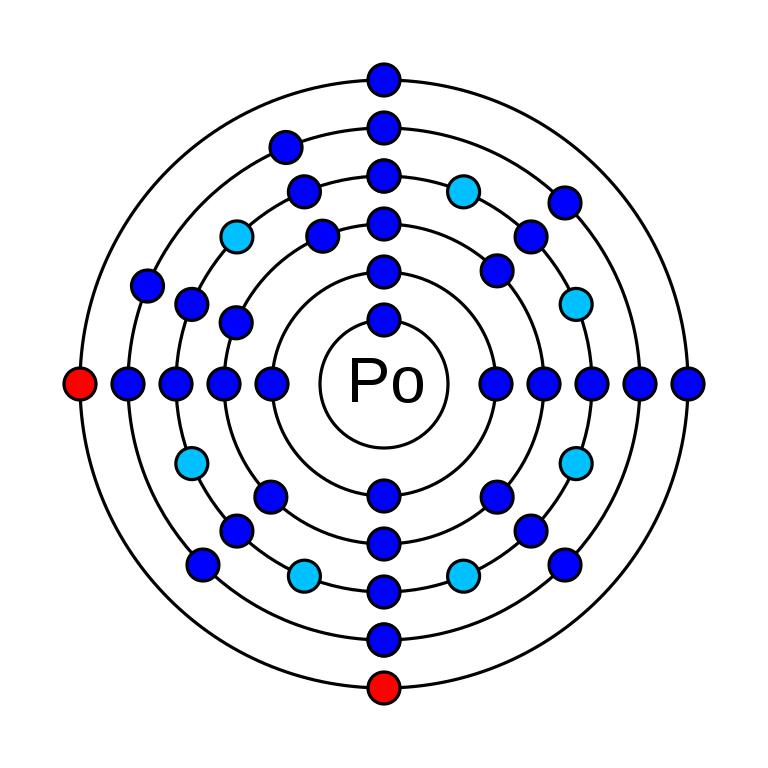
Polonium is a chemical element that has the atomic number 84. Its atomic weight is 208.98 g/mol and it is represented by the symbol Po. It is a p-block element that can be found in period 6 and group 16 of the periodic table. In an electrically neutral atom of there are 84 electrons and protons. It has 125 neutrons. The electronic configuration of Polonium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p4. Its electro negativity is 2.0 (Pauling scale) and its oxidation states are −2, +2, +4, +5, +6.
Polonium has 42 isotopes, all of them are radioisotopes. The longest half-life radioisotope is polonium-210 which has a half-life of 138.376 days.
Polonium exists in two allotropic forms alpha and beta. It is in solid-state at STP and has a density of 9.196 g/cm3 for alpha allotropic form and 9.398 g/cm3 for beta allotropic form. Its melting point is 527 K ?(254 °C, ?489 °F) and its boiling point is 1235 K ?(962 °C, ?1764 °F). Polonium in its pure form has a silvery appearance but tarnishes on exposure to air. It is showing similar chemistry with tellurium and bismuth. It is readily soluble in dilute acids and slightly dissolved alkalis solutions.
It was discovered by Pierre and Marie Curie in 1898 and was isolated by Willy Marckwald in 1902. Polonium was named after Poland (Latin Polonia) which was the homeland of Marie Curie.
Polonium is a very rare element in nature. It is produced as decay products in the decay chain of U-238 and U-235. It can be found in uranium ores.
Polonium beryllium oxide is used as a neutron source for nuclear weapons. Po-210 is used as an atomic heat source for the moon rovers to keep its component warm. It is also used in satellite-like Kosmos 84 and 90 satellites. It is also used to remove static charges in photographic plates paper rolls and sheet plastics etc. Polonium spark plugs were once used from 1940 to 1953.
What type of radiation is emitted when polonium-212 forms lead-208?
Polonium-212 has a half-life of 299 nanoseconds. It will emit alpha (α) particle forming lead- 208.
When was polonium discovered?
Polonium was discovered in 1898 by Pierre and Marie Curie. It was isolated by Willy Marckwald in 1902. Polonium was named after Poland (Latin Polonia) which is the homeland of Curie.
What does the 218 in polonium-218 represent?
The number 218 is the atomic mass of the Polonium-218 isotope. It has 84 protons and 134 neutrons (84+134=218).
To what element does polonium-208 (atomic number 84) decay when it emits an alpha particle?
If Polonium-208 emit alpha particle it will decay into lead-204 (Pb-204). And on beta emission, it will decay into bismuth-208.
How many valence electrons does polonium have?
Polonium has 6 valence electrons and it is in 6s2 6p4 orbitals.