Table of Contents
4. How many valence electrons does radium have?
What is radium?
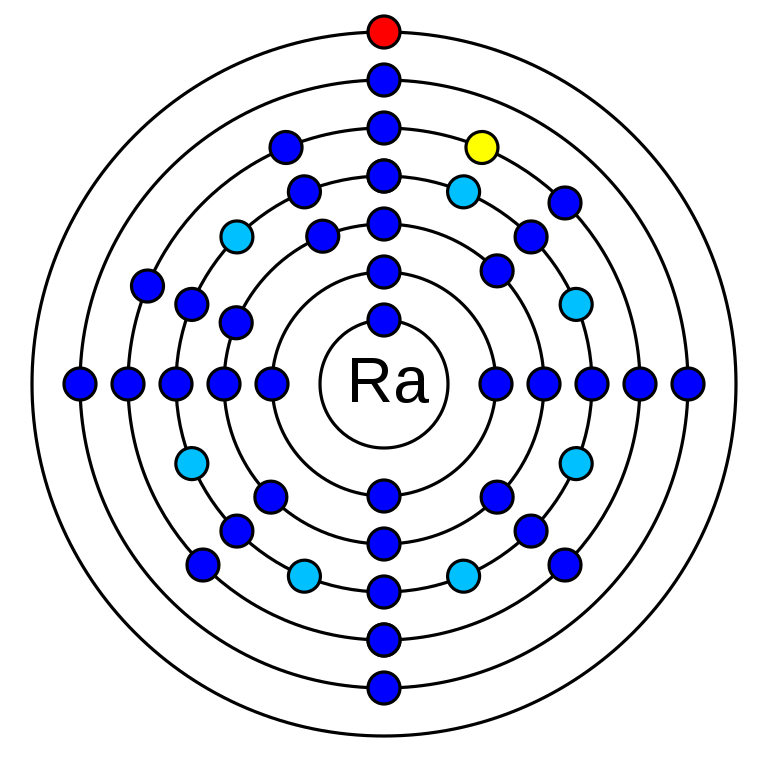
radium is a chemical element that has the atomic number 88. Its mass number is 226 and it is represented by the symbol Ra. It is an s-block element and is an alkaline earth metal. In the periodic table, it can be found in group 2 and period 7. There are 88 electrons in an electrically neutral atom of Radium. The electronegativity of Radium is 0.9 (Pauling scale) and its oxidation state is +2. Radium electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s2. Radium has no stable isotope. It has 33 radioisotopes. The longest-lived isotope of Radium is Ra-226 which has a half-life of 1600 years.
At STP radium is solid and has a density of 5.5 g/cm3. Its melting point is 973 K ?(700 °C, ?1292 °F) (disputed) and its boiling point is 2010 K ?(1737 °C, ?3159 °F). It is the only radioactive element of the alkaline earth mrtals group. In pure form, Radium has a silvery-white metallic appearance. It was discovered by Pierre and Marie Curie in 1898 and was isolated by Marie Curie in 1910.
Radium is produced as a result of natural decay of thorium and uranium isotopes. In small amounts, it is found in uranium ores such as uraninite and to some extend in thorium minerals also.
Radium has some application in research and Industries. It is used in Atomic, molecular, and optical physics research. In 2013 the United States Food and Drug Administration approved Radium isotope Ra-223 for use in medicine as a cancer treatment of bone metastasis. It is still used as a source of radiation in industrial radiography for finding flaws in metallic parts.
Who discovered radium?
Radium was discovered by Pierre and Marie Curie in 1898 and was also isolated by Marie Curie in 1910.
What is radium used for?
Radium was once used as an additive in food items, toothpaste, and hair cream. In the 1920 and 30s, it was used in medicine for cancer treatment in the form of radium chloride and radium bromide. It was then banned and replaced with cobalt-60. In the 1900s biologists used radium for mutation studies. It is used in Atomic, molecular, and optical physics research. In 2013 the United States Food and Drug Administration approved Radium isotope Ra-223 for use in medicine as a cancer treatment of bone metastasis. It is still used as a source of radiation in industrial radiography for finding flaws in metallic parts.
How many valence electrons does radium have?
Radium has 2 valence electrons. They are in 7s orbital (7s2).