Table of Contents
2. Which element has the highest melting point tantalum rhenium osmium hafnium?
6. How many protons does rhenium have?
7. Where was rhenium discovered?
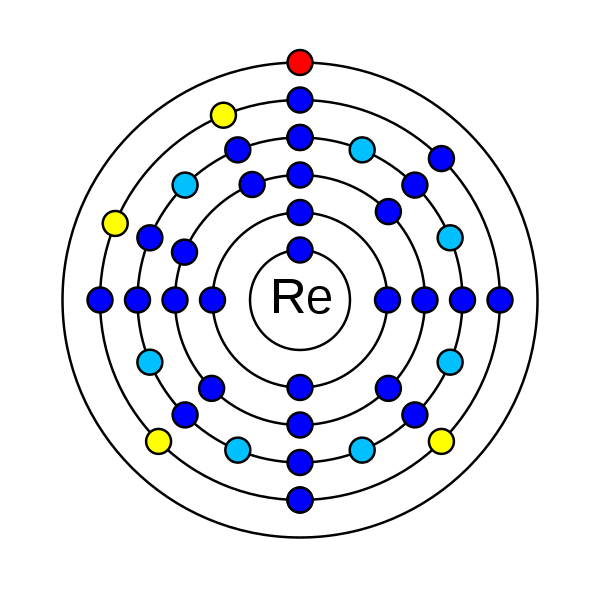
8. How many electrons does rhenium have?
Rhenium is a chemical element that has the atomic number 75. Its atomic mass is 186.207 g/mol and has been represented by the symbol Re. In the periodic table, it is in period 6 and group 7. It is a d-block transition metal. There are 75 electrons in an electrically neutral atom of Rhenium. The electronic configuration of Rhenium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d5 6s2. Its electronegativity is 1.9 (Pauling scale). The oxidation state of rhenium are −3, −1, 0, +1, +2, +3, +4, +5, +6, +7. Rhenium has one stable isotope rhenium-185 and has 33 radioisotopes. Rhenium-187 a radioisotope has more abundance than the rhenium-185 stable isotope. This phenomenon can be observed in indium and tellurium.
It has a silvery-greyish appearance. At STP rhenium is solid and has a density of 21.02 g/cm3. The melting point of Rhenium is 3459 K ?(3186 °C, ?5767 °F) and its boiling point is 5903 K ?(5630 °C, ?10,170 °F). Rhenium was first discovered by Walter Noddack, Ida Noddack, Otto Berg in 1925. And it was first isolated by Walter Noddack, Ida Noddack in 1928. Rhenium was named after the river Rhine.
It is a very rare element and has an average concentration of 1ppb in the earth's crust. Rhenium can not be found free in nature but with other rare metals in ores. It could be found in minerals like molybdenite and rheniite. China is the leading producer of rhenium, followed by Chile, the United States, Peru, and Poland. Total world production is not exceeding 60-70 tons/year.
Some of the following are the applications. It is used in making superalloys. Rhenium alloys are used in jet engines such as F-16, F-15 and F-35. Rhenium tungsten alloys are used in thermocouples to measure temperatures up to 2200 °C. Rhenium filaments are used in photoflash lamps, mass spectrometers and ion gauges because of their low vapour pressure and high melting point. Rhenium-platinum alloy as a catalyst is used extensively in the petroleum industry. It is used in the conversion of Napthas into high octane number products. Rhenium catalyst is used in the olefin metathesis. Rhenium has a high chemical poisoning resistance from Phosphorus, Sulfur and Nitrogen which could be used in hydrogenation reactions. Rhenium radioisotopes such as rhenium-188 and rhenium-186 are used in the treatment of liver cancer.
Which element has the highest melting point tantalum rhenium osmium hafnium?
The melting point of each of the elements is as follows.
Tantalum melting point is 3290 K ?(3017 °C, ?5463 °F)
Rhenium melting point is 3459 K ?(3186 °C, ?5767 °F)
Osmium melting point is 3306 K ?(3033 °C, ?5491 °F)
Hafnium melting point is 2506 K ?(2233 °C, ?4051 °F)
From the above data, we concluded that Rhenium has the highest melting point.
Where is rhenium found?
In the earth's crust, it has an average concentration of about 1 ppb. Rhenium is found in the minerals such as molybdenite and rheniite which is commercially very important. The main producers are China, Russia, Chile, the USA and Poland etc.
Who discovered rhenium?
Rhenium was discovered by Walter Noddack, Ida Noddack, Otto Berg in 1925. They detected it in platinum mineral columbite. It was predicted by Dmitri Mendeleev in his periodic table. It was first isolated by Walter Noddack, Ida Noddack in 1928.
What is rhenium used for?
Some of the following are the applications.
- It is used in making superalloys. Rhenium alloys are used in jet engines such as F-16, F-15 and F-35.
- Rhenium tungsten alloys are used in thermocouples to measure temperatures up to 2200 °C.
- Rhenium filaments are used in photoflash lamps, mass spectrometers and ion gauges because of their low vapour pressure and high melting point.
- Rhenium-platinum alloy as a catalyst is used extensively in the petroleum industry. It is used in the conversion of Napthas into high octane number products.
- Rhenium catalyst is used in the olefin metathesis. Rhenium has a high chemical poisoning resistance from Phosphorus, Sulfur and Nitrogen which could be used in hydrogenation reactions.
- Rhenium radioisotopes such as rhenium-188 and rhenium-186 are used in the treatment of liver cancer.
How many protons does rhenium have?
Rhenium has 75 protons. It has an atomic mass of 186.207 g/mol. It has 111 neutrons in its nucleus.
Where was rhenium discovered?
Rhenium was discovered in Germany by three german scientists Walter Noddack, Ida Noddack, Otto Berg in 1925. It was named after the River Rhine.
How many electrons does rhenium have?
In an electrically neutral atom of rhenium, there are 75 electrons.