Table of Contents
4. How many protons does thorium have?
What is thorium?
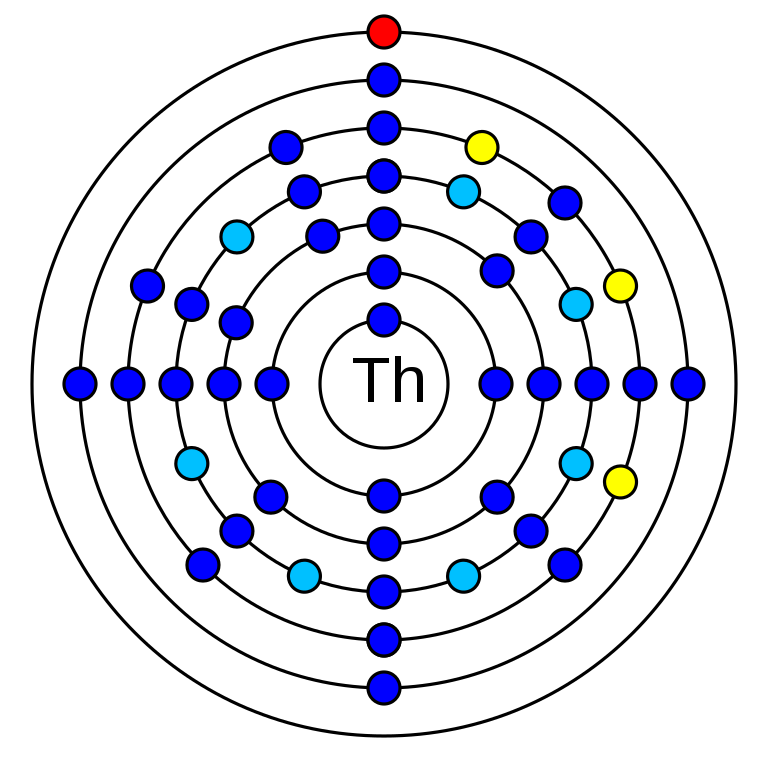
Thorium is a chemical element that has the atomic number 90. It has an atomic mass of 232.037 g/mol and it is represented by the symbol Th. Thorium is the second member of the actinide series. It is an f-block element and it is in period 7. In an electrically neutral atom of Thorium, there are 90 electrons. Its electronegativity is 1.3 (Pauling scale) and its oxidation states are +1, +2, +3, +4. Thorium electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f0 6d2 7s2. Despite an f-block element it has an anomalous [Rn]6d27 s2 electron configuration The ionization energy required for the 1st electron is 587 kJ/mol and for the 2nd electron is 1110 kJ/mol. Thorium has no stable isotope there are some seven naturally occurring. The long-lived isotope of thorium is Th-232 which has a half-life of 1.405×1010 years.
Thorium has a silvery color when exposed to air it turns into black color. At STP Thorium is solid and it has a density of about 11.7 g/cm3. Its melting point is 2023 K ?(1750 °C, ?3182 °F) and its boiling point is 5061 K ?(4788 °C, ?8650 °F).
Thorium was discovered by Swedish chemist Jöns Jakob Berzelius in 1829. It was named after the Norse god of thunder Thor.
Thorium has an abundance of about 8.1 ppm in Earth's crust. A major source of Thorium is ore like Monazite which has deposits worldwide. Countries which has Monazite deposits in India, South Africa, Brazil, Australia, and Malaysia. Other ores which contain Thorium in small amounts are thorianite, Allanite, and Thorite.
Thorium has some applications. It was used in the gas mantle from 1885 onward in the form of thorium oxide. It is used as an alloying element in TIG welding. 90% platinum and 10% thorium alloy are used catalysts for oxidizing ammonia to nitrogen oxides. Thorium dioxide is used in glass which helps increase its refractive index. In multilayered optical coatings, thorium tetrafluoride is used as an anti-reflection. It can be used for nuclear energy and can satisfy world energy demands for longer because it is more abundant than uranium.
Who discovered thorium?
Thorium was discovered by Swedish chemist Jöns Jakob Berzelius in 1829. It is named after the Norse god of the thunder Thor.
Why don't we use thorium?
Using thorium as a source of nuclear energy has an advantage and a disadvantage. It is more abundant than uranium and can provide nuclear energy for a long time. Thorium is used in molten salt reactors. It is producing uranium-233 through neutron-induced fission of a nuclide. Uranium-233 can also be used in the same way as U-235 and Pu-239.
The disadvantage of Thorium use is that it producing strong gamma emitters. Which is dangerous and very difficult to reprocess.
How many protons does thorium have?
Thorium has 90 protons in its nucleus.