Table of Contents
5. How many neutrons does tungsten have?
6. How many valence electrons does tungsten have?
7. When was tungsten discovered?
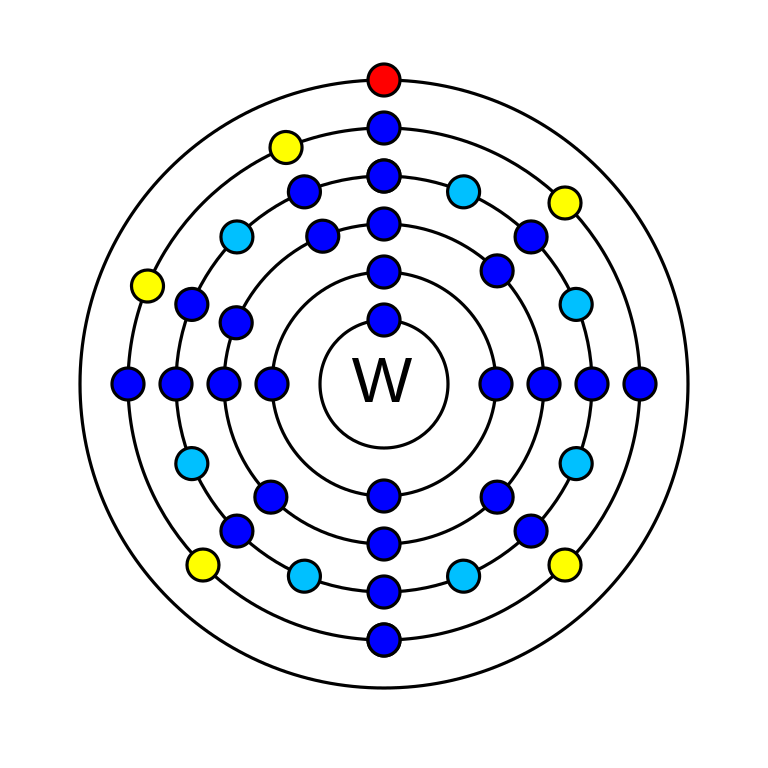
Tungsten is a chemical element that has the atomic number 74. It has an atomic mass of 183.84 g/mol and it is represented by the symbol W (wolfram). It is a d-block element. In the periodic table, it could be found in period 6 and group 6. There are 74 electrons, 74 Protons and 110 neutrons in an electrically neutral atom of tungsten. The electronic configuration of tungsten is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d4 6s2. It has oxidation states −4, −2, −1, 0, +1, +2, +3, +4, +5, +6 and its electronegativity is 2.36 (Pauling scale). Thirty-three radioisotopes of tungsten have been known. Naturally, there are five isotopes of tungsten among which one is radioisotope (W-180) the rest are stable.
Tungsten has two crystalline forms, alpha and beta. At STP it is solid and has a density of 19.3 g/cm3. It has a white greyish colour. Of all the metals in pure form, tungsten has the highest melting point. Its melting point is 3695 K ?(3422 °C, ?6192 °F) and its boiling point is 6203 K ?(5930 °C, ?10706 °F).
It was discovered and first isolated by two Spanish chemists Juan José Elhuyar and Fausto Elhuyar in 1783.
Tungsten is found in different ore with a different ratio with other elements. Economically important ores of tungsten are hübnerite, ferberite, wolframite and scheelite. The world's reserves of tungsten are in China, Canada, Australia, Vietnam, Rwanda, Congo and Russia. China is the leading producer and exporter.
Half of the tungsten production is used in hardening materials and in tungsten carbide. It is used in cutting tools like circular saws, knives, and drills etc. It is also used in the jewellery industry for the production of rings. Tungsten is has a good erosion resistance and a high melting point. This makes it a good choice for the inner wall (plasma-facing material ) of the nuclear fusion reactors. It is used in the Joint European Torus test reactor. Tungsten is used in the formation of nanowires. Nanowires have been studied since 2002. Due to its high melting point tungsten is used in high-temperature electronics. Tungsten(IV) sulfide has a high melting point and it is used as a high-temperature lubricant. Due to its hardness and high melting point, it is used in the formation of different alloys. Some of them are used in missiles such as a submarine-launched ballistic missile. As well as used in applications like rocket nozzles and turbine blade etc.
What is tungsten used for?
- Industrial uses:
Half of the tungsten production is used in hardening materials and tungsten carbide. It is used in cutting tools like circular saws, knives, and drills etc.
- Jewellery:
It is also used in the jewellery industry for the production of rings. It has almost the same density as gold. Rings made of tungsten are shiny and lustrous.
- plasma-facing material:
Tungsten has a good erosion resistance and a high melting point. This makes it a good choice for the inner wall (plasma-facing material ) of the nuclear fusion reactors. It is used in the Joint European Torus test reactor.
- Nanowires:
Tungsten is used in the formation of nanowires. Nanowires have been studied since 2002. Due to its high melting point tungsten is used in high-temperature electronics.
- Lubricant:
Tungsten(IV) sulfide has a high melting point and is used as a high-temperature lubricant.
- Military applications:
Due to its hardness and high melting point, it is used in the formation of different alloys. Some of them are used in missiles such as a submarine-launched ballistic missile. As well as used in applications like rocket nozzles and turbine blade etc.
What colour is tungsten?
Tungsten has a greyish white appearance.
How strong is tungsten?
The Mohs hardness of tungsten is 7.5. For that comparison, Platinum is 3.5. Osmium is 7, gold is 2.5 and diamond is 10. Pure tungsten metal has the lowest coefficient of thermal expansion. It has a high melting point and tensile strength because of its strong metallic bond.
How many neutrons does tungsten have?
Tungsten has 110 neutrons.
How many valence electrons does tungsten have?
Tungsten has six valence electrons.
When was tungsten discovered?
Tungsten was discovered and isolated in 1783 by two Spanish chemists Juan José Elhuyar and Fausto Elhuyar. But it was named by Swedish chemist Torbern Bergman in1781.