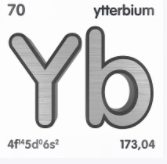
Ytterbium is a chemical element that has the atomic number 70. Its atomic mass is 173.04 g/mol and represented by the symbol Yb. It is in the lanthanide group and period 6 of the periodic table. It is an f-block element the last electrons are accommodated in the 4f14 6s2 orbitals. The electronic configuration of the Ytterbium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d0 6s2. There are 70 electrons, 70 protons and 103 neutrons in an electrically neutral atom of ytterbium. The electronegativity of ytterbium is 1.1 (Pauling scale). Its oxidation state are 0, +1, +2, +3.
Ytterbium has twenty-seven radioisotopes and seven naturally occurring stable isotopes. Ytterbium-174 is the most abundant isotope of ytterbium.
Ytterbium has a silver-white appearance. It is a soft ductile chemical element when pure. It quickly dissolved with mineral acids. Also reacts with cold water but slowly. It has three allotropes alpha, beta and gamma. Ytterbium is paramagnetic above 1 kelvin. At STP it sold and has a density of about 6.90 g/cm3. Its melting point is 1097 K ?(824 °C, ?1515 °F) and its boiling point is 1469 K ?(1196 °C, ?2185 °F).
Ytterbium was discovered by Swiss chemist Jean Charles Galissard de Marignac in 1878 and was first isolated by Carl Auer von Welsbach in 1906.
Ytterbium can be found with other rare earth elements in several minerals. Minerals that have ytterbium are euxenite, xenotime and monazite sand. Countries having deposits of these minerals are China, Sri Lanka, the US, India, Brazil, and Australia.
In quantum computing charged ion 171Yb+ is used in trapped-ion qubits. It is also used in lasers such as Yb3+ ion which is used as a doping material in active laser media mostly in double-clad fibre lasers (DCF) and in solid-state lasers. Ytterbium is also used as a doping agent in stainless steel, enhancing strength and other mechanical properties. Ytterbium is used in high-stability atomic clocks. Atomic clocks based on ytterbium atoms hold a record for stability with ticks stable to within less than two parts in 1 quintillion. Ytterbium is a source of gamma rays. Ytterbium isotope Yb-169 is used for gamma radiation in portable X-ray machines.
What is ytterbium used for?
Some of the following are the uses and applications of Ytterbium.
- Quantum Computing:
In quantum computing charged ion 171Yb+ is used in trapped-ion qubits.
- Used as a source of Gamma rays:
Ytterbium is a source of gamma rays. Ytterbium isotope Yb-169 is used for gamma radiation in portable X-ray machines.
- Used in Lasers:
It is also used in lasers such as Yb3+ ion which is used as a doping material in active laser media mostly in double-clad fibre lasers (DCF) and in solid-state lasers.
- Used as a Doping agent:
Ytterbium is also used as a doping agent in stainless steel, enhancing strength and other mechanical properties.
- Stable atomic clocks:
Ytterbium is used in high stability atomic clocks. Atomic clocks based on ytterbium atoms hold a record for stability with ticks stable to within less than two parts in 1 quintillion.
Where is ytterbium found?
Ytterbium is a rare earth element that is found with other rare elements in minerals like monazite, euxenite and xenotime. The main mining areas of these minerals are in China, Brazil, Australia, India, Sri Lanka and the US.
When was ytterbium discovered?
Ytterbium was discovered in 1878 by Swiss chemist Jean Charles Galissard de Marignac. In 1905 it was isolated by Austrian chemist Carl Auer von Welsbach.
How to pronounce ytterbium?
Try it. (i-TUR-bee-?m).
How many neutrons does ytterbium have?
Ytterbium has 103 electrons. But the number is different in different isotopes. Ytterbium has seven stable isotopes.
How many electrons does ytterbium have?
In an electrically neutral atom of ytterbium, there are 70 electrons and 70 protons.