Table of Contents
1. Covalent Bond (The Sharing of Electrons):
2. Different Types of Covalent Bonds:
Covalent Bond (The Sharing of Electrons):
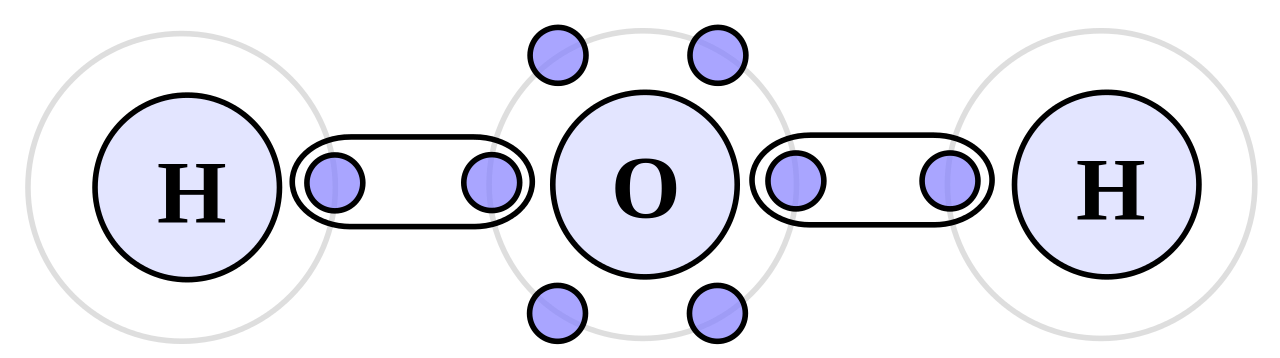
A covalent bond is a chemical bond that forms by sharing electrons pairs between atoms. This type of bond is found between elements that are non-metallic. This involves electron sharing but not transfer. During covalent bond formation no charged particles are produced but only groups of atoms called molecules. Atoms in molecules attained a stable electronic configuration through covalent bonding. Covalent bonding is more common in organic chemistry.
Consider two Chlorine atoms each requires one electron to complete its orbit through octet rule and attain noble gas electronic configuration. Each atom has seven electrons in its outermost shell. Both will share one electron through covalent bonding. This will complete their last shell and attain a stable electronic configuration.
Different Types of Covalent Bonds:
A covalent bond can be a single bond or it can be multiple bonds. In a single covalent bond, the bond is a strong Sigma (σ) bond. Sigma bond is the result of head-on overlapping of two orbitals of two different atoms. This is a case for a single covalent bond. If there are more covalent bonds between two atoms, the first bond is a Sigma (σ) bond the remaining are Pi (π) bonds. There is one Pi (π) bond and one sigma bond in a double covalent bond. In a triple covalent bond, there are two Pi (π) bonds and one Sigma (σ) bond. Keep in mind that the Pi (π) bond is weaker than the Sigma (σ) bond. Pi (π) bonds are formed when two orbitals sidewise overlap.