Table of Contents
2. What is an element in science?
4. Which element has the smallest atomic radius?
6. What is the center of an atom called?
7. Where are the electrons located in an atom?
Elements and Atoms
The world we are living in is made of different substances. These substances are a combination of fundamental materials which are called elements. An element can not be broken down into simpler substances. An element is a pure form of one type of atom. These atoms which is making an element have the same number of protons in their nucleus. Atoms of one element are identical but from atoms of another element.
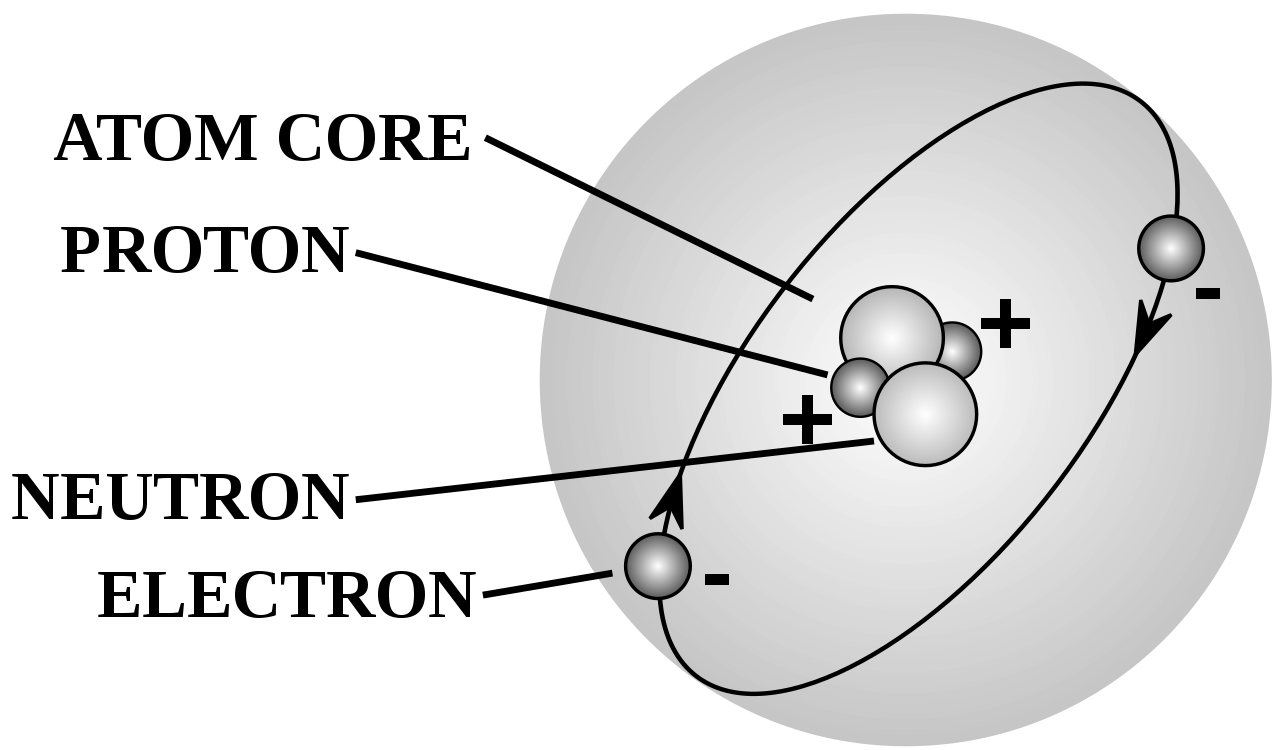
An atom is the smallest possible particle of a chemical element. Every state of matter (Solid, Gas, Liquid, and Plasma) have consisted of atoms or ions. Atom is an extremely small particle for an ordinary measurement ( about 100 picometers). Each atom has consisted of neutrons, protons, and electrons. Neutrons and protons are in the nucleus of an atom which makes about 99.94% of an atom's mass. The electrons are circulating around the nucleus in different shells and orbitals depending on their energies. Proton is positively charged, a neutron is neutral and an electron is a negatively charged particle. If an atom has an equal number of protons and electrons then the atom is electrically neutral.
The nuclear force between proton and neutron keeps them together in the nucleus. There is an electromagnetic force between proton and electron. The nuclear force is greater than the electromagnetic force. But when the electromagnetic force exceeds than nuclear force the atom goes through Nuclear decay.
FAQs
What is an element in science?
A pure substance which is consisted of similar atoms (Having the same number of protons).
What is element 115?
In the periodic table element, 115 is Moscovium (Symbol Mc).
Which element has the smallest atomic radius?
The element which has the smallest atomic radius is Helium. It is because the electrostatic force of attraction between electrons and protons is very high. Another clue we can get from ionization energy. Helium has the highest ionization energy.
What is an atom?
An atom is the smallest possible particle of a chemical element. Every state of matter (Solid, Gas, Liquid, and Plasma) have consisted of atoms or ions. Atom is an extremely small particle for an ordinary measurement ( about 100 picometers). Each atom has consisted of neutrons, protons, and electrons.
What is the center of an atom called?
The Center of the atom is called the nucleus or nuclei. It consists of protons and neutrons.
Where are the electrons located in an atom?
Electrons are in shells, sub-shells, and orbitals of an atom. These are places where the probability of finding an electron is high.