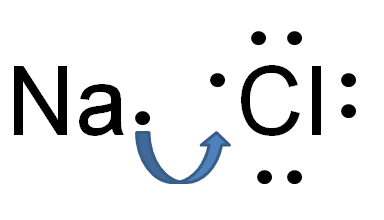Table of Contents
4. What is the fundamental difference between covalent and ionic bonding?
Ionic Bonding:
It is a type of chemical bonding in which two differently charged ions are attracting each other through electrostatic force. It can also happen between two atoms having a high difference of electronegativity. Usually, ionic bonding occurs between metals and non-metals. Ionic bonding occurs in ionic compounds. Overall the ionic compounds are neutral which are held together by the electrostatic force of attraction.
In ionic bonding, an atom that is less electronegative its electron is pulled by the atom which is more electronegative. There is not a complete loss of an electron but sharing of one electron. The electrostatic force of attraction develops which keeps both atoms together. The atom which partly loses electrons are mostly metals and they are called cations. Those atoms which pull electrons are mostly nonmetal because they are more electronegative. When an atom gains an electron it is called Anion.
Ionic compounds have a high melting point. This is because of the strong electrostatic force of attraction between ions. Ionic compounds do not conduct electricity in the solid-state but they are good conductors of electricity in molten form.
Why Does Ionic Bond form?
Ionic bonding occurs between atoms that have a greater difference in electronegativity. Those elements which have a low affinity for electrons mostly metal try to lose electrons. They have low ionization energy, giving some of the electrons to achieve a stable electronic configuration. On the other hand, there are elements that had a greater electron affinity and high ionization energy. These are mostly nonmetal, which attains stable electronic configuration by gaining electrons. The electrostatic force of attraction keeps these atoms (Cation and anion) together via ionic bonding.
Take the example of table salt which is sodium chloride. It has sodium which is a metal and has low electron affinity. By losing one electron it attains the noble gas electronic configuration. On the other hand, chlorine which is a non-metal has a high electron affinity and high ionization energy. Chlorine can achieve a stable electronic configuration by gaining an electron from sodium. Sodium loses its outermost electron to Chlorine becoming cation and anion. Both cation and anion start attracting each other because of the electrostatic force of attraction between oppositely charged particles. this is called ionic bonding.
Na + Cl → Na+ + Cl− → NaCl
FAQs
What is ionic bonding?
It is a type of chemical bonding in which cation and anion are attracting each other via electrostatic force of attraction. In ionic bonding between two-atom, one atom loses its electron to another but not completely. There is certain degree of hold which keeps both atoms together.
What is the fundamental difference between covalent and ionic bonding?
A covalent bond is sharing of two electrons between two atoms something mutual. While in an ionic bonding there is only one electron involved between two highly electronegative and electropositive atoms. One loses its electron to the other while electrostatic force keeps them together.