Table of Contents
3. How many neutrons does bismuth have?
4. How many valence electrons does bismuth have?
5. When the isotope bismuth-213 emits an alpha particle, what new element results?
6. How many protons are in an atom of bismuth?
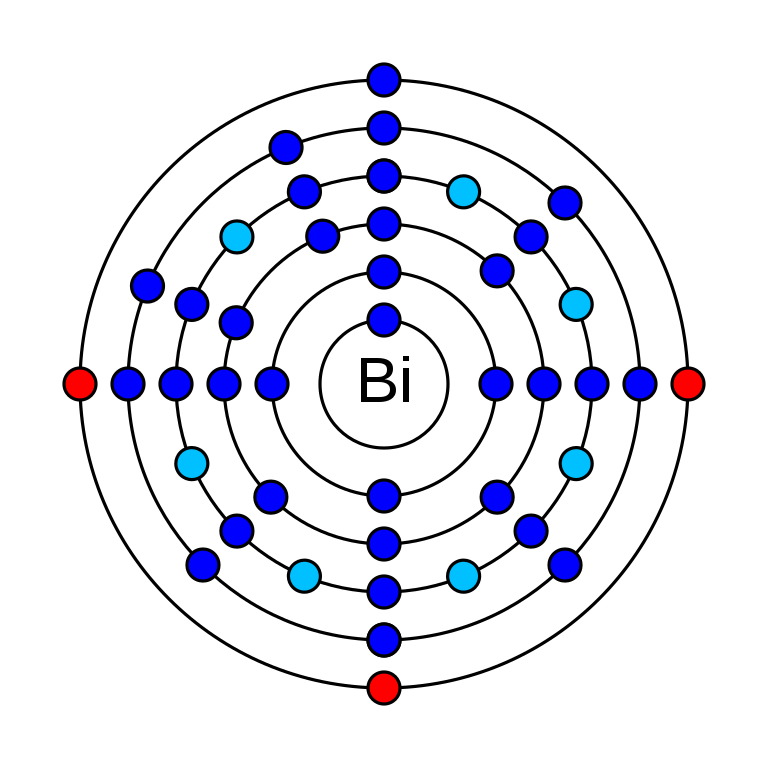
Bismuth is a chemical that has an atomic number (Z) 83. It has an atomic mass of 208.980 g/mol and it is represented by the symbol Bi. It is in group 15 and period 6 of the periodic table. It is a p-block element and its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p3. In an electrically neutral atom of Bismuth, there are 83 protons and electrons. It has 126 neutrons in its nucleus. Its oxidation states are −3, −2, −1, +1, +2, +3, +4, +5. The electronegativity of Bismuth is 2.02 (Pauling scale). Bismuth has 41 isotopes in which one Bi-209 has a very long half-life which is safe for use. Its radioactivity was theoretically predicted. In 2003 it was experimentally demonstrated in the lab that Bi-209 is a radioisotope with a half-life of 2.01×1019 years (3 Bq/Mg).
Bismuth was confused with tin and lead in the early days because of its resemblance. Later on, it was Claude François Geoffroy who differentiate it from lead and tin. It has a brownish silver appearance. Pure bismuth is denser in the liquid phase than solid. Naturally, it is more diamagnetic than any other metal. At STP it is solid and has a density of 9.78 g/cm3 but in the liquid phase, its density increases to 10.05 g/cm3. Its melting point is 544.7 K ?(271.5 °C, ?520.7 °F) and its boiling point is 1837 K ?(1564 °C, ?2847 °F).
Bismuth is extracted from ores like bismite and bismuthinite. In Earth crust's it is twice abundant as gold. The main producer of bismuth is China, Vietnam, Mexico, Australia and Japan.
Bismuth and its compounds have a wide range of applications some of which are following.
Bismuth oxychloride is used in cosmetics for nail polishes and eye shadows. In ancient Egypt, the Egyptians used bismuth oxynitrate in cosmetics.
It is used in alloys and is an alternative for lead. Lead is poisonous for wildlife. Lead is replaced by bismuth alloy which is non-toxic and functions similarly.
In medicine, bismuth is used extensively. Bibrocathol is used for eye infections which has bismuth. Bismuth subsalicylate ( Pepto-Bismol) is used for antidiarrheal treatment. It is also used for the treatment of cadmium poisoning and some gastro-intestinal diseases like shigellosis.
It is used as a catalyst in making acrylic fibres.
Bismuth subnitrate is used as a pigment in paint.
Bismuth telluride is an excellent thermoelectric material its diodes are used in CPU coolers and in mobile refrigerators. It is also used in semiconductors and in infrared spectrophotometers and detectors. Bismuth germanate is used in gamma-ray and X-ray detectors.
What is bismuth used for?
Bismuth and its compounds have a wide range of applications some of which are following:
- Semiconductors:
Bismuth telluride is an excellent thermoelectric material its diodes are used in CPU coolers and in mobile refrigerators. It is also used in semiconductors and in infrared spectrophotometers and detectors.
Bismuth germanate is used in gamma-ray and X-ray detectors.
- Cosmetics:
Bismuth oxychloride is used in cosmetics for nail polishes and eye shadows. In ancient Egypt, the Egyptians used bismuth oxynitrate in cosmetics.
- Alloy and alternative of Lead:
It is used in alloys and is an alternative for lead. Lead is poisonous for wildlife. Lead is replaced by bismuth alloy which is non-toxic and functions similarly. It is also used in water pipe valves instead of Lead, in residential and commercial buildings
- Medicine:
In medicine, bismuth is used extensively. Bibrocathol is used for eye infections which has bismuth. Bismuth subsalicylate ( Pepto-Bismol) is used for antidiarrheal treatment. It is also used for the treatment of cadmium poisoning and some gastro-intestinal diseases like shigellosis.
- Catalyst:
It is used as a catalyst in making acrylic fibres. Also used electrocatalyst in the conversion of CO2 to CO.
- Pigment and Paint:
Bismuth subnitrate is used as a pigment in paint.
How many neutrons does bismuth have?
In a stable Bismuth atom, it has 126 neutrons.
How many valence electrons does bismuth have?
Bismuth has 5 valence electrons. They are in 6s2 and 6p3 orbitals.
When the isotope bismuth-213 emits an alpha particle, what new element results?
Bismuth-213 will convert into Thallium-209 on alpha decay. Bismuth-213 has a half-life of 45 minutes. On β− decay it will convert onto polonium-213.
Bi-213------> Tl-209 + α
How many protons are in an atom of bismuth?
In a stable Bismuth atom, there are 83 protons in its nuclei.