Table of Contents
4. When was thallium discovered?
5. How many valence shell electrons does an atom of thallium have?
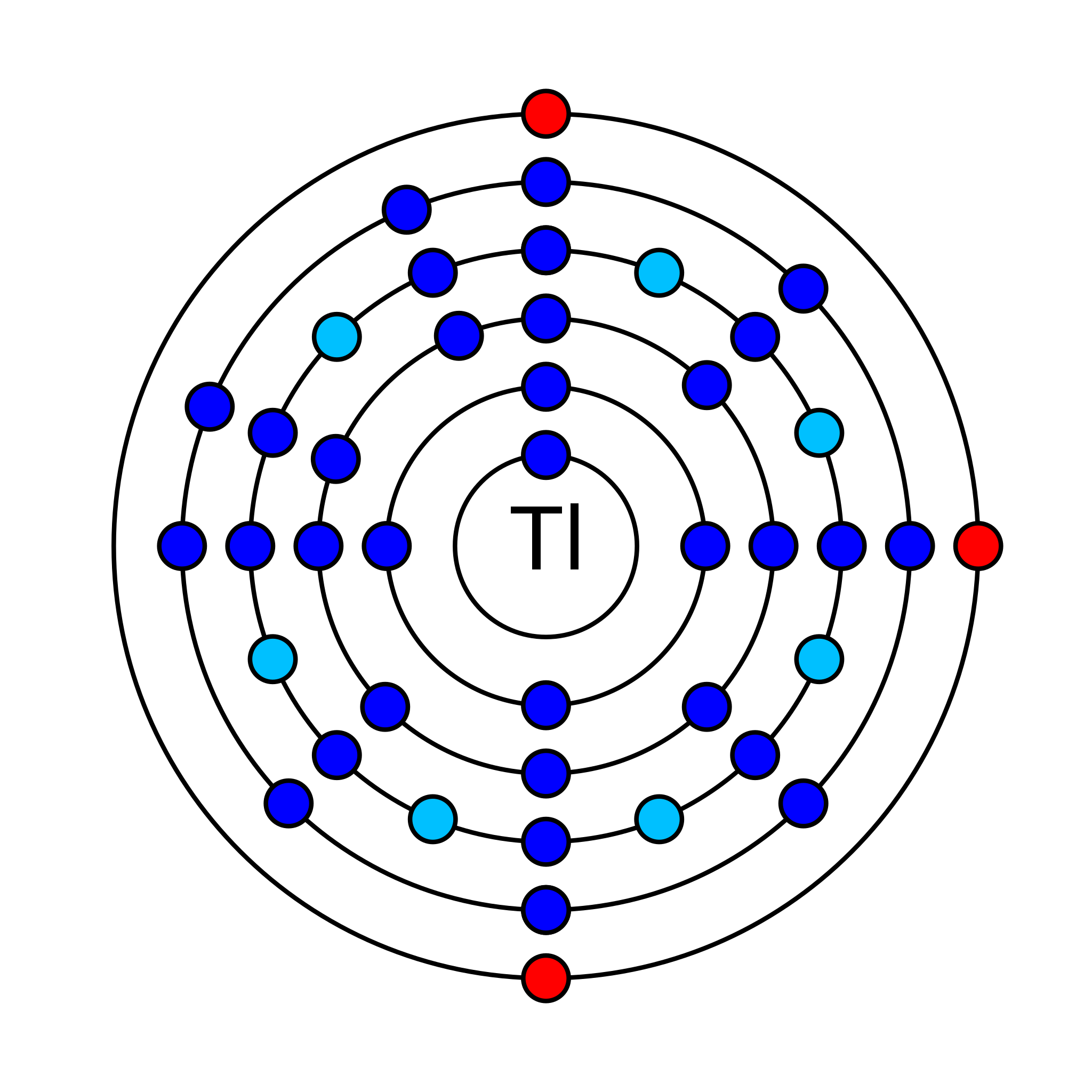
Thallium is a chemical element that has an atomic number (Z) 81. It has an atomic mass of 204.38 g/mol and it is represented by the symbol Tl. It is a p-block element in group 13 and period 6 of the periodic table. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p1. In an electrically neutral atom of thallium, there are 81 electrons and 123 neutrons. Its oxidation states are −5, −2, −1, +1, +2, +3 and its electronegativity is 1.62 (Pauling scale). It has 41 isotopes of which two are stable Tl-203 and Tl-205. Tl-205 is the most abundant isotope with an average abundance of 70.5%.
It has a silvery-white colour resembling tin it tarnishes on exposing to air. At STP it is in the solid phase and has a density of about 11.85 g/cm3. Its melting point is 577 K ?(304 °C, ?579 °F) and its boiling point is 1746 K ?(1473 °C, ?2683 °F).
Thallium was discovered by William Crookes and Claude-Auguste Lamy independently at the same time in 1861.
Thallium has an average concentration of 0.7 mg/kg in Earth's crust. Thallium is obtained as a by-product from the smelting of sulfide ores of Zinc, lead and copper. Minerals which are rich in Thallium are lorándite, crookesite and hutchinsonite.
Thallium has a wide range of applications. The radioisotope Tl-203 was once the main substance to use for nuclear cardiography. Then it was replaced with technetium-99m. Thallium cuprate is used as a high-temperature superconductor and the research is still going on the superconductivity of mercury-doped thallium-cuprate. Thallium selenide is used in bolometer for infrared detection. Thallium doping is also used in gamma radiation detection devices. Thallium iodide and bromide crystals are used in infrared optical devices. It is also used in high-density glasses. Historically thallium sulfate is used as rat poison and ant killer because it is odorless and tasteless. later on it was prohibited due to safety concerns.
What is thallium 201?
Thallium-201 is a radioisotope of thallium. It is used in the thallium stress test in nuclear medicine. On electron capturing it will produce Hg-201 daughter isotope. Thallium-201 has a half-life of 7 hours.
Thallium consists of 29.5% Ti-203 and 70.5% Ti-205. what is the relative atomic mass of thallium?
Given data:
1st Isotope mass = 203. Abundance = 29.5%
2nd Isotope mass = 205%. Abundance = 70.5%
The formula for relative atomic mass is:
Relative atomic mass = (isotope 1 mass × isotope 1 abundance + isotope 2 mass × isotope 2 abundance + …) ÷ 100.
Now for put these values in the above equation.
Relative atomic mass = 203 x 29.5 + 205 x 70.5 ÷ 100
Relative atomic mass = 204.41(g/mol)
What is thallium used for?
Thallium has a wide range of applications. Some of the following are:
- Nuclear Medicine:
The radioisotope Tl-203 is used in nuclear cardiography (Thallium stress test).
- Superconductors:
Thallium cuprate is used as a high-temperature superconductor and the research is still going on the superconductivity of mercury-doped thallium-cuprate.
- Electronics:
Thallium selenide is used in bolometer for infrared detection. Thallium doping is also used in gamma radiation detection devices.
- Optics:
Thallium iodide and bromide crystals are used in infrared optical devices. It is also used in high-density glasses.
- Rat Poison:
Historically thallium sulfate is used as rat poison and ant killer because it is odourless and tasteless. later on, it was prohibited due to safety concerns.
When was thallium discovered?
It was discovered in 1861 by William Crookes and Claude-Auguste Lamy independently.
How many valence shell electrons does an atom of thallium have?
Thallium has three valence electrons in its outer shell. Two are in 6s-orbital and one is in 6p-orbital.