Table of Contents
In Bohr's atomic theory when an electron moves?
In Bohr's atomic theory what happens when one electron jumps from one shell to another?
What about Bohr's atomic theory was partially correct and was also refined by other scientist?
Bohr's Atomic model/Theory:
This theory was in response to Rutherford's atomic model. It overcomes some of the drawbacks of Rutherford atomic theory.
Postulates:
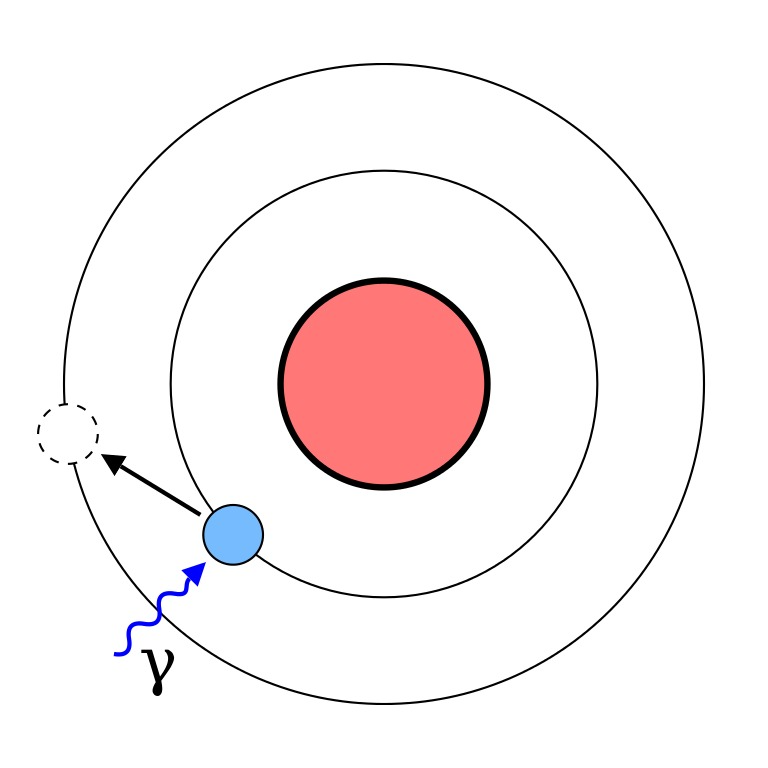
1. The electrons are revolving around the nucleus in fixed circular paths called shell or orbits.
2. The energy of an electron is fixed in a particular orbit. As long as the electron is present in the particular orbit it does not gain or lose energy.
3. The energy of the electron is proportional to its distance from the nucleus. Greater the distance of an electron from the nucleus higher will be its energy.
4. When an electron jumps from a higher energy level to a lower it loses energy. But when an electron jumps from a lower to higher energy level it gains energy.
ΔE = E2 - E1 = hf
ΔE = energy difference of electron in lower and higher level.
E2 = is the energy of an electron at a higher level.
E1 = is the energy of an electron in the lower level.
h = plank's constant
f = frequency of the radiation.
5. Electrons cannot stay in between two orbits it will always present in one of the orbit.
6. Electron will move in that orbit which has the angular momentum (mvr) of the electron is an integral multiple of h/2π.
mvr = n(h/2π)
m = mass of electron
v = velocity
r = radius of an orbit
n = number of shell (n = 1, 2, 3......)
h = plank's constant ( 6.63 × 10-34 J s)
7. Electron present in the first orbit has less energy than the electron in the next orbit.
In Bohr's atomic theory when an electron moves?
When an electron gains or loses energy it moves from its shell (orbit). When an electron jumps from a higher energy level to a lower it loses energy. But when an electron jumps from a lower to higher energy level it gains energy.
In Bohr's atomic theory what happens when one electron jumps from one shell to another?
When an electron jumps from a higher energy level to a lower it loses energy. But when an electron jumps from a lower to higher energy level it gains energy.
What about Bohr's atomic theory was partially correct and was also refined by other scientist?
Partially Bohr's atomic theory was correct and several refinements were proposed one of such is Bohr–Sommerfeld model. It suggests that electrons travel in elliptical orbits around a nucleus instead of a circular path.