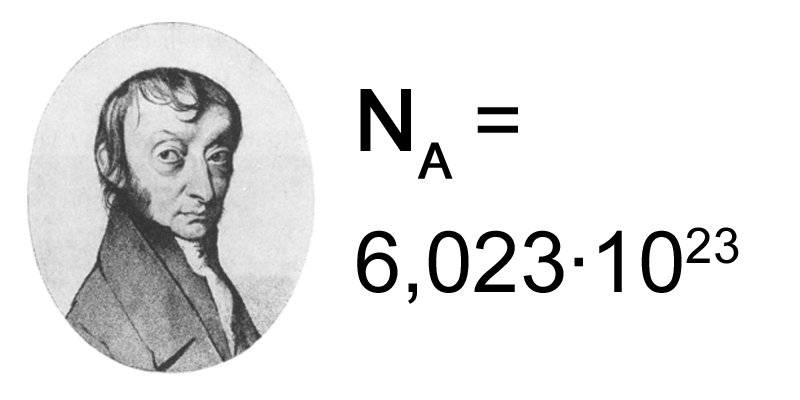Table of Contents
How many particles are in one mole?
Mole:
In the international system of units (SI) Mole is a unit for the amount of substance. If you take exactly 6.02214076×1023 particles of atoms, ions, or molecules that would be equal to one mole. On the other hand, if you take 12 grams of carbon (C-12 isotope) that would have 6.02214076×1023 atoms in it. This means it would be equal to one mole. But this amount of particles ( 6.02214076×1023) is in 2 grams of Hydrogen. Now the question is why 2 grams of Hydrogen but 12 grams of carbon (C-12)? The answer to this is that Hydrogen atoms are lighter and smaller so 6.02214076×1023 is found in 2 grams. Remember it is about the number 6.02214076×1023, not the mass.
This number 6.02214076×1023 is called the Avogadro number. In other words, a mole is essentially a count of particles. The amount of substance is the number of moles in the sample. But what is molar mass? The molar mass of a substance is the mass of one mole of that substance. It is numerically equal to its relative atomic mass, given in grams per mole.
There is a relationship between the mass of a sample of an element and the number of moles it contains:
mole = mass/molar mass or Mol = m/M
Quick question:
How many moles are there in 30g of carbon?
You can easily find out through the above formula.
Given data:
mass = 30g
Molar mass = 12g/mol
Now put these values in the equation and do the math. Your answer will be 2.5 mol.
Do it for 30 g of Magnesium?
There is a relationship between the Avagadro constant ( symbol L) whose value is 6.02214076×1023/mol number of moles and the number of atoms (particles).
Number of atoms = Avagardro constant x number of moles
N = L x n
We can find out the number of atoms in a specific amount of mole.
For Example:
How many atoms are there in 1.5 moles of Hydrogen?
Given Data:
L = 6.02214076×1023/mol
n = 1.5 mol
now put these values in the above equation.
N = 6.02214076×1023/mol x 1.5 mol
N = 9.0 ×10'23 Hydrogen atoms.
FAQs
What is a mole?
In the international system of units (SI) Mole is a unit for the amount of substance. one mole is the exact 6.02214076×1023 particles.
How many grams in a mole?
It depends on the sample. One mole of carbon (C-12) has 12 grams of carbon. While one mole of Hydrogen has 2 grams of Hydrogen. This is because Hydrogen atoms are smaller and lighter than Carbon atoms.
How many molecules in a mole?
There are 6.02214076×10'23 molecules in one mole.
How many atoms are in a mole?
There are 6.02214076×1023 atoms in one mole.
How many particles are in one mole?
There are 6.02214076×1023 particles in one mole.